The Crucial Role of Stem Cell Peptides in Anti-Photoaging
Glen Alvin1*, Yuriy Nalapko1,3 and Mike K.S Chan1,2,3,4
1European Wellness Biomedical Group, Malaysia, Germany
2FCTI Research & Development GmbH – Germany
3The European Society for Preventive, Regenerative & Anti-Ageing Medicine, Athens, Greece
4Lincoln University College, Malaysia
*Corresponding author: Glen Alvin, European Wellness Biomedical Group, Malaysia, Germany
Citation: Alvin G, Nalapko Y and Chan KS M. (2024) The Crucial Role of Stem Cell Peptides in Anti-Photoaging. J Stem Cell Res. 5(2):1-10.
Received: August 28, 2024 | Published: September 16, 2024
Copyright© 2024 genesis pub by Alvin G, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributedunder the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Introduction
Aging is a natural process, divulging the gradual decay of a living organism [1]. Aging is not a disease, and anti-aging therapy aims to lag, stop, or reverse the aging process and its associated effects, particularly on the skin, like wrinkles and skin fragility [2]. Factors like genes, race, and diet influence the rate and severity of intrinsic aging [1]. As the body ages, its ability to maintain structural integrity and repair damages progressively declines [3]. Skin ageing is characterised by deteriorating skin density and elasticity, failing to maintain a youthful appearance [4]. Skin ageing, especially in women, reduces their self-esteem and confidence [3].
The natural, intrinsic aging process occurs over time, starting in the late thirties [3]. This slow process involves various mechanisms simultaneously, bringing alterations to skin tissue [3]. Changes in the epidermis include changes in the shape and texture of the skin [3]. In the dermis, the biochemical metamorphosis causing aged skin unveils a decrease in extracellular matrix (ECM) proteins, increased collagen degradation, the loss of melanocytes, keratin and decreased fibroblasts [5-3]. A mishanterous revamp to the mechanical interaction between fibroblasts and the ECM results in functional abnormalities [6]. The skin surface appears paler, more transparent, and more fragile [3]. Fine wrinkles appear on its surface, becoming atrophic, drier, and thinner from the loss of subcutaneous fat [3].
Photoaging, a significant and damaging aspect of skin aging, results from intrinsic and extrinsic factors [7]. Research suggests that a staggering 80% of facial aging is attributed to photoaging [7-8]. The culprits behind this process, including ultraviolet (UV) radiation, infrared radiation, chemical smoke, dust, and haze, can have severe and long-lasting effects on the skin [8]. With its profound influence, UV radiation stands out as the most impactful [8]. Prolonged exposure to UVB can lead to erythema, sunburn, oxidative damage, inflammation, and, in severe cases, skin cancer [8].
When it comes to treating photoaging, there are two main categories: preventive and reversal [12]. Preventive measures like sunscreens are the most efficient and essential in avoiding photodamage [9]. On the other hand, reversal treatments involve cosmeceuticals for facial rejuvenation, an area of research that has seen robust development and funding from some of the forerunners in the cosmetic industry [9]. Stem cell research is at the forefront of this field [9].
Topical retinoids remain the gold standard for antiaging therapy [6]. Ascorbic acid (vitamin C) is another popular antiaging treatment [6]. Ascorbic acid eliminates 'Reactive oxygen species' (ROS) and is a cofactor required to synthesise procollagen and elastin, preserving the skin's physiological state [6]. Topical α- hydroxy acids like glycolic or lactic acid stimulate Glycosaminoglycans (GAGs) and collagen synthesis, which improve the histologic quality of elastic fibres in photoaged skin [6]. Lasers and light therapy induce the wound-healing process, trigger an immune cell influx and concentrate inflammatory mediators, ultimately fabricating a new ECM [6]. Radiofrequency (RF) medical devices and High-intensity focused ultrasound (HIFU) are newer technologies that promote neocollagenesis and neoelastogenesis, improving skin laxity [6].
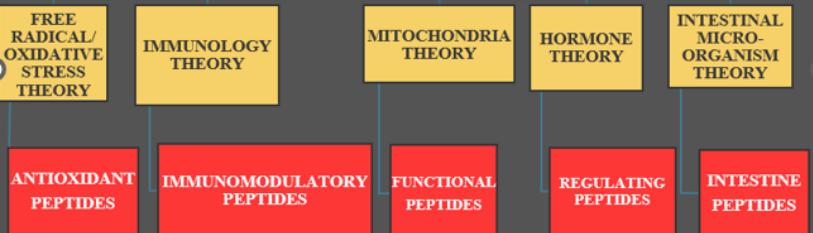
The anti-aging market is expected to grow at an approximate 8% compound annual growth rate, reaching a value of USD 271 billion in 2024 [10] (Figure 1).
Figure 1: Some theories of aging and their interventional peptides.
Pathophysiology of Photoaging
Photoaging, or extrinsic skin aging, results from chronic UV ray exposure [3]. Its incidence starts in young adults and increases with age [3] noted that 72% of men between the ages of 30 and 39 and 42% of women between the ages of 20 and 29 have experienced photoaging in Australia [3]. Darker skin tones and frequent sunbathing pose a higher risk for photoaging [3]. Extrinsic aging from chronic UV radiation exposure not only impacts the aesthetic aspect of the skin but also imparts a strong correlation with skin cancer from genetic mutation [7,11].
Histologically, epidermal photodamage is characterized by alteration in thickness, with alternating areas of atrophy and hypertrophy and some degree of nuclear atypia in Keratinocytes (KC) [10]. These changes reflect the dysregulation of KC growth exposed to UVR [12]. Fibroblast-derived cytokines, including keratinocyte growth factor (KGF), Epidermal growth factor (EGF), and Hepatocyte growth factor (HGF), stimulate KC growth [12]. Mast and inflammatory cells also produce several cytokines contributing to the pathological changes in sun-damaged skin [12]. KGF and chemokine receptor-2 (CXCR-2) levels in human KC decrease after UVB irradiation, thus decreasing the ability of the epidermis to regenerate and to respond to an increased dermal synthesis of KGF and an epidermal overproduction of IL-8and Gro-α [12]. Melanocytes are affected by cytokines derived from KC AND dermal cells, including fibroblasts and inflammatory cells [12]. Melanocyte growth-promoting activity, responsible for pigmentary anomaly observed in photo-damaged skin, is triggered by β-Fibroblast growth factor (βFGF), Endothelin-1 (ET-1), HGF, and Stem cell growth factor (SCF), as well as the activity of melanocyte tyrosinase [12]. Vessels in photoaged skin are most likely obliterated and so have the standard horizontal plexus architecture associated with cutaneous ships in the upper dermis [13]. There is a considerable reduction in small vessels in sun-protected aged skin, especially in the upper dermis [13]. The papillary dermis of photoaged skin exhibits a significant decrease in vessel size, vascular number and cutaneous area covered by the blood vessels [13].
The dermis primarily comprises the ECM and fibroblasts [6] Collagen is the major component of ECM, while other ECM components include elastic fibers, glycosaminoglycans (GAGs), and proteoglycans (PGs) [6]. Structural and quantitative changes in collagen fibers, where the fibrils are fragmented and coarsely distributed, are the main remodelling seen in aged skin, compared to young skin, which has abundant, tightly packed, and well-organized intact collagen fibrils [6]. Aged skin suffers from increased collagen degradation, reduced collagen biosynthesis, and dysfunctional collagen homeostasis, which results in net collagen loss [6]. This process leads to clinical changes observed in aged skin [6]. Elastin, an extracellular matrix protein, imparts elasticity and resilience to connective tissue, giving the skin tautness and suppleness [7]. Elastic fibers comprise soluble tropoelastin molecules cross-linking by lysyl oxidase (LOX) [6]. Each elastic fiber is made up of numerous elastic microfibrils (such as fibrillin, fibulin, and microfibrillar-associated glycoproteins (MAGPs], and latent TGF-β -binding proteins (LTBPs)) and amorphous elastin [6]. In young skin, the elastic fibres adopt a highly ordered architecture, with perpendicularly oriented fibrillin-rich microfibrils at the papillary dermis and large-diameter elastic fibres at the reticular dermis [6]. Vascular smooth muscle cells and fibroblasts aid in elastin synthesis [7]. This physiological phenomenon typically ceases with the onset of puberty, coinciding with the body's maturation [7]. The protease elastase causes the degradation of elastin fibers, characterised by accumulated disorganised elastic fibers throughout the dermis in a “solar elastosis” process [6]. The primary mechanism responsible for elastic fiber degradation in photoaging is the activation of MMPs5: MMPs-2, -[3,9,12,13] catabolise elastic fibers [6]. MMP-12 is a human macrophage metalloelastase, the most active protease in elastin degradation [6]. Age-related elastic fiber alterations are also influenced by fixation with lipids and calcium, besides the proteolytic degradation of elastic fibers [14] (Figure 2).
Melanin is the primary protective mechanism against UV in the skin [7]. Following UVB exposure, melanocytes in the skin's basal layer generate excessive melanin, leading to skin pigmentation [7]. Melanin synthesis is facilitated by a series of oxidation reactions mediated by the enzyme tyrosinase (TYR), where TYR expedites the synthesis and transportation of the pigment [7]. Glycosaminoglycans (GAGs) or mucopolysaccharides are linear polysaccharides located in the dermis [5]. There are 6 types of GAGs, including chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), heparin (HP), and hyaluronic acid (HA) [6]. All GAGs, except HA, contain sulfate substituents at different positions on the polysaccharide chain and are heavily glycosylated on their proteoglycan (PG) core proteins [6]. GAG chains contain numerous negatively charged carboxyl and sulfate groups, essential in maintaining the water content in dermal tissue [6]. Specific GAG quantities, like HS and CS, decrease during intrinsic aging, while KS and DS increase [6]. The total sulfated GAGs increase in photoaged skin, with CS demonstrating an increase at the solar elastosis region [6]. Fibroblasts mainly synthesise Dermal HA and are abundant in the papillary dermis [6]. HA has a vital impact on skin moisture due to its strong ability to bind water [15]. HA can also support skin volume expansion and turgidity and contribute to the diffusion of metabolites and nutrients [15]. HA crosslinks with ECM proteins, like collagen, stemming increased tissue stiffness and a unique shock-absorbing role [6]. In intrinsically aging skin, HA binding proteins (HABPs) are reduced [6]. HABPs trigger several intracellular signalling pathways that regulate the proliferation, migration, and differentiation of HA [6]. Studies demonstrate that the HA content around solar elastosis skin significantly increases, confirming that HA contents are increased in photoaged skin [6]. UV irradiation induces HA synthase (HAS), indicating that unknown regulatory mechanisms are responsible for the abnormal accumulation of nonfunctional proteins in photoaged skin [6].
Figure 2: The schematic diagram of the process of photoaging.
The occurrence of low-grade inflammation is called Inflammaging [16]. Inflammaging leads to an imbalance between the inflammatory response mediators in favour of the proinflammatory response represented by cytokines and oxidative stress [16]. The Transforming growth factor-β (TGF-β) signalling stirs the regulation of cell growth, differentiation, migration, immune/inflammatory responses, and angiogenesis [6-11]. TGFβ is upregulated and activated in photoaging skin, inducing excessive metalloproteinases (MMPs), which are a family of ubiquitous endopeptidases, pro-inflammatory cytokines, tumour stimulatory effects and prolonged infiltration of neutrophils, leading to progressive collagen degradation and aberrant elastic fibers that contribute to ECM destruction [6-11]. The major sources of MMPs are epidermal keratinocytes and dermal fibroblasts in the skin, although they are also synthesised by the endothelial cells and immunocytes [6]. Chronic UV exposure galvanises TGFβ1/Smad3 signalling activation and metalloproteinase-induced collagen degradation and photo-inflammation in photoaging [11]. The changes in collagen synthesis and degradation are the main contributor to these visible changes in aging skin. At the same time, DNA damage and decreased ability to repair damaged skin cells also lead to mutations in skin cells [3]. These molecular changes are manifested as lentigo solaris, actinic keratosis, seborrheic keratosis, pigmentary disorders, telangiectasia, wrinkles, lack of elasticity, sagging and even skin cancer [3-9]. Wrinkles and skin laxity are the typical phenomena of skin aging and result from progressive atrophy of the dermis, where there is a decline in ECM volume, particularly collagen55. The levels of MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, MMP-11, MMP-13, MMP-17, MMP-26, and MMP-27 are raised in aged skin, mainly attributed to ROS [6].
Photoaging is a cumulative process dependent on the intensity of UV radiation. When the intensity of exposure is too low to induce skin damage, it merely activates AP1 and p53, which represses TGFβ expression and restrains integrin-dependent latent TGFβ activation [11]. Exposure to prolonged UV irradiation also triggers an explosion of cytokine activation, such as NF-κB, which unshackles inflammatory response via the release of IL-1, IL-6, cyclooxygenase-2 (COX-2), and TNF-α1. NF-κB mediates UV irradiation responses and is responsible for the upregulation of MMP-1 and MMP-3 in dermal fibroblasts [6]. The inflammatory ambush by cytokines leads to mitochondrial impairment, heightened levels of ROS and orchestrates a cyclic release of inflammatory mediators [7]. Oxidative stress, distinguished by an imbalance between oxidation and antioxidant mechanisms, contributes purposefully to photoaging [7]. In physiological conditions, a limited quantity of reactive oxygen species (ROS) endows the body with immunological benefits [7]. ROS also initiates the activation of the MAPK signalling pathway [8]. MAPK is a family of serine and tyrosine kinases containing extracellular signal-regulated kinase (ERK), c-Jun N- terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) that regulates cell proliferation, differentiation, apoptosis, and inflammation [8]. UVB fuels the Phosphorylation of MAPK, which activates the c-Jun transcription factor and the nuclear factor kappa B (NF-κB), which in turn switches on the transcription factor activation protein-1(AP-1) complex [17]. The AP-1 and NF-κB pathways energise MMPs and heme oxygenase-1 (HO-1) to degrade collagen and elastic fibers, leading to wrinkle formation [17]. Excessive ROS production induced by UV radiation disrupts the cellular REDOX capacity, thus distorting the equilibrium of skin homeostasis [7]. The mRNA levels of p38, JNK, ERK, MMP- 1, and MMP-3 in HaCaT cells were significantly upregulated by UVB irradiation [18]. ROS generated upon UV irradiation also builds up malondialdehyde (MDA) levels. It impairs the antioxidant defence systems, such as superoxide dismutase (SOD) and catalase (CAT), thus fostering cellular structure and component damage, leading to photoaging [19]. ROS generated in photoaging skin increases MMP expression and inhibits TGF-β signalling, leading to collagen fragmentation and decreased collagen biosynthesis [6]. This hinders the mechanical interaction between fibroblasts and the ECM, consequently evolving smaller-sized dermal fibroblasts [6]. Aged fibroblasts beget more ROS, which further increases the expression of MMPs and inhibits TGF-β signalling, creating a positive feedback loop that accelerates dermal aging [6].
Nuclear factor-erythroid 2-related factor 2 (Nrf2) protects the skin from UV-related oxidative damage and cellular dysfunction [4]. The Nrf2/antioxidant response element (ARE) pathway is the principal defence mechanism of the skin against oxidative stress [4]. This pathway regulates the expression of antioxidant genes, such as heme oxygenase-1 (HO-1), quinone oxidoreductase 1 (NQO1), CAT, and SOD [4]. When induced by antioxidants, Nrf2 disrupts Kelch-like ECH-associated protein 1 (Keap1), the primary molecule responsible for its negative regulation, followed by rapid nuclear translocation and transactivation of ARE- associated genes [8]. This process promotes ROS scavenging, maintains redox homeostasis, suppresses inflammation, and repairs damaged DNA, thereby supporting the survival of skin cells [8]. TNF-α is a widely distributed proinflammatory factor in the body and mediates various physiological and biochemical responses such as inflammation, apoptosis, and immune response. In the skin, TNF-α promotes the synthesis of the cellular inflammatory factor IL-6 via the COX-2 signalling pathway in response to UVB irradiation [8] (Figure 3).
Figure 3: Signs of photoaging on the human face.
Intrinsic aging is an internal process that occurs with advancing age and is characterised by fine wrinkles and a thinning epidermis [5]. In contrast, extrinsic aging is characterised by deep wrinkles, skin laxity, and hyperpigmentation, mainly set off by chronic sun exposure [6]. Collagen fibers are a significant component of the ECM [6]. In human skin, type I collagen makes up 80 to 90% of the total collagen, while type III makes up 8 to 12% [6]. Elastic fibers are another fibrous element that makes up the dermal ECM [6].
Elastic fibers return the skin to its normal configuration after being stretched or deformed [6]. The other components of the ECM are proteoglycans (PGs) and glycosaminoglycans (GAGs) [6]. The other dermal components include fibroblasts, which are responsible for synthesising and degrading fibrous and amorphous ECM proteins [5]. Their interaction with the environment influences the dermal aging process [6]. The dermis also boasts the presence of immune cells like histiocytes, mast cells, and dermal dendrocytes, as well as endothelial cells and skin appendages [6].
Adult tissue stem cells support tissue homeostasis and repair throughout life [25]. The visible signs of aging are a consequence of altered stem cell behaviour and reduced tissue maintenance and regeneration [25]. Failure of efficient stem cell functioning prompts changes in cell cycle progression, decreased bioenergetic efficiency, increased DNA damage, and epigenetic alterations [20].
Peptide Therapy in Photoaging
Glutathione was the first biological anti-aging peptide synthesised in the laboratory, and its development boomed the anti-aging industry, fuelling the masses' craving for healthy aging, especially for the skin [26].
Peptides are short amino acid chains linked by peptide bonds that can functionally alter skin physiology [5, 21]. They are the macromolecules that are the backbone of living organisms [21]. Both peptide and protein moieties are characterised by stereoselectivity, a hallmark feature determining their biomolecular processes, which range from catalysis to self-assembly [22]. Peptides are involved in intercellular communication, where they deliver bioactive molecules, like proteins, nucleic acids, and lipids, to recipient cells, thereby influencing various cellular processes [23]. Transferring these bioactive molecules plays a crucial role in mediating and regulating multiple physiological and pathological processes, such as proliferation, migration, and differentiation, thus influencing tissue homeostasis and repair [23]. Short- chained peptides penetrate cell membranes more deftly to activate signalling pathways regulating the differentiation of gene expression through various ways, such as interacting with histone proteins, changing gene accessibility for transcription, regulating gene methylation status, activating/inhibiting their expression, or directly interacting with DNA [24]. Peptides possess inherent biocompatibility, the ability to traverse physiological barriers, and minimal immunogenicity [18]. Their specificity, faculty to mimic natural biological functions, precision in targeting specific proteins and receptors, and few side effects compared to traditional drugs make them attractive for treating various diseases [25]. The other virtues of peptides include deep tissue penetration, low immunogenicity, efficient internalisation into cells, minimal toxicity to the internal organs, and easy manipulation using chemicals compared to antibodies [25]. Therapeutic peptides are categorised based on their pharmacological activities, such as replacing deficient proteins, augmenting existing pathways, providing novel functions, or interfering with specific molecules or organisms [29].
Peptides are versatile molecules that can be utilised in many different skin conditions in cosmeceuticals [27]. Peptides manoeuvre skin ageing by regulating collagen turnover and scheme specific neurotransmitters to decrease age-induced wrinkles [27]. The primary mechanism behind peptides in photoaging is to increase collagen synthesis and restore lost ECM to prevent wrinkles [5]. Fibroblasts are pivotal dermal cells that synthesise and secrete collagen and elastin [15]. As we age, the number of fibroblasts dwindles, compromising the production of collagen fibers and causing loss of skin elasticity [15]. Peptides regulate fibroblastic activity in the governance of ECM components, mainly through signal peptides [5]. The elastin peptide, Val-Gly-Val-Ala-Pro-Gly (VGVAPG) hexapeptide, binds to elastin‐binding proteins and thus stimulates fibroblast proliferation [15]. Bioactive peptides can mitigate the physiological alterations associated with photoaging, including oxidative stress, inflammatory response, the abnormal expression of matrix metalloproteinase, hyaluronidase, and elastase, and excessive melanin synthesis [7]. Anti-aging peptides augment skin cell viability and proliferation, diminish pigmentation, mitigate tissue inflammation, enhance skin barrier functionality, and provide support to the skin [1]. Like all other peptides, anti-aging peptides are classified according to their mechanisms of action, encompassing signal peptides, carrier peptides, neurotransmitter inhibitor peptides, and enzyme inhibitor peptides [1]. Peptides are regarded as biological compounds with the potential to alleviate the early aging phenomenon of skin, strengthen the skin barrier function, and protect against UV radiation [28]. Understanding the function and networks of peptides leads to their utilisation as supplements or therapeutics, supporting physiological and pathological conditions [26].
Elastin Peptides (Eps) regulate a plethora of biological activities, including angiogenesis, cell chemotaxis, proteinase synthesis, tumour invasion, proliferation, and ROS production [6]. An example of an EPs reaction in the skin is the activation of the MEK/ERK signalling cascade, which mediates cell proliferation via fibroblast [6]. EPs also protect dermal fibroblast from ceramide-induced apoptosis, allowing ceramide accumulation in living cells [6].
Zinc‐α2‐glycoprotein (ZAG) peptides enhance the mRNA expression of filaggrin and reduce the mRNA expression of genes associated with trans‐epidermal water loss (TEWL) and inflammatory factors [4]. They fend off skin aging by improving the skin's barrier function and improving skin density and elasticity [4]. ZAG peptides lessened Senescence-associated β-galactosidase (SA‐β‐gal), which prompts skin aging by collagen and other dermal connective tissue breakdown [4]. They shrink the expression of MMP2 and MMP9 while inducing the expression of TIMP1 in keratinocytes and dermal fibroblast quantity [4]. Applying ZAG peptide improved skin density, elasticity, and wrinkles in a clinical setting [16].
Peptides like aminoacyl tRNA synthase complex-interacting multifunctional protein 1 (AIMP1)-derived polypeptide, better known as the Sh-oligopeptide, have clinically proven to have positive effects on type I collagen synthesis in human fibroblasts [28]. Cyanocuprin is a peptide that induces collagen and elastin synthesis by increasing fibroblastic activity. It is regulated by transcription factors such as Smad and mitogen-activated protein kinase (MAPK) pathways [28]. The tetrapeptide GEKG rouses fibroblasts to form collagen and smooth skin [28]. GHK-Cu (glycyl-L-histidyl-L-lysine-Cu) is a peptide that promotes skin remodelling, wound healing, regeneration, antioxidant and anti-inflammatory effects [28].
The diminution of communication between the mitochondria and nucleus due to mitochondrial dysfunction is a well-recognized cause of aging [27]. MOTS-c is a 16-amino-acid mitochondrial-derived peptide encoded in the 12S rRNA region of the mitochondrial genome [29]. They execute cellular functions that benefit age-related diseases, including Diabetes, Cardiovascular diseases, Osteoporosis and skin aging [29]. At the cellular level, this peptide elevates NAD (+) levels, exerts glycolytic effects via sirtuin 1
(SIRT1), favourably sways the folate/methionine cycle and restricts methionine metabolism [29] confirmed that the MOTS-c peptide amplified skin collagen by reducing IL-6, a critical inflammatory factor of MMP1 in the dermis [29]. This peptide also lowered inflammation, which led to an increase in dermal collagen [23].
Palmitoyl Tripeptide-1 (PAL-GHK) and Palmitoyl hexapeptide-12 (Pal-KTTKS) are biosimilars that were previously known as “Palmitoyl oligopeptide” [10]. PAL-GHK are matrikine-mimetic peptides that stimulate fibroblast activity, which increases matrix protein and glycosaminoglycan synthesis in the dermis [10]. (PAL-GHK) stimulates collagen synthesis in a human fibroblast while preventing its degradation after exposure to UVA light [10]. Lintner et al. mentioned that a clinical trial demonstrated that Palmitoyl Tripeptide-1 increased the skin thickness of 23 healthy female subjects with minimal adverse events [10]. Palmitoyl hexapeptide-12 is an elastin fragment that stimulates collagen and elastin synthesis, fibronectin, and glycosaminoglycans, improving skin elasticity and firmness [10].
Peptides can also be fabricated for precision medicine [26]. Decades of research and development have successfully enabled peptides to be moulded to perform specific functions [26]. An excellent example is thymus peptides, which restore T-cell function [26].
Conclusion
Aging is a biological spectacle involving all tissues, organs, and systems, but it is most evident on the skin. With age, the skin undergoes molecular changes that govern its morphology and, ultimately, its function. The current approaches to treat and prevent photoaging leave much to be desired. Hence, a new paradigm is required for antiaging therapy.
Peptides are relatively novel bioactive compounds in the aesthetic world [10]. Their chemical and biological properties encourage the industry to develop innovative compounds continually [10]. Peptide therapy efficacy and safety profile make them an excellent option for treating photoaging [25].
References
- Zhang X, Zhuang H, Wu S, Mao C, Dai Y, et al. (2024) Marine Bioactive Peptides: Anti-Photoaging Mechanisms and Potential Skin Protective Effects. Curr Issues Mol Biol. 46(2):990-1009.
- Wei M, Qiu H, Zhou J, Yang C, Chen Y, et al. (2022) The Anti-Photoaging Activity of Peptides from Pinctada martensii Meat. Mar Drugs. 20(12):770.
- Ke Y, Wang XJ. (2021) TGFβ Signaling in Photoaging and UV-Induced Skin Cancer. J Invest Dermatol. 141(4S):1104-1110.
- Cui B, Wang Y, Jin J, Yang Z, Guo R, et al. (2022) Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid Med Cell Longev.2022:6037303.
- Shin JW, Kwon SH, Choi JY, Na JI, Huh CH, et al. (2019) Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int J Mol Sci. 20(9):2126.
- Baud S, Duca L, Bochicchio B, Brassart B, Belloy N, et al. (2013) "Elastin peptides in aging and pathological conditions" Biomal Concepts. 4(1):65-76.
- Liu Z, Li Y, Song H, He J, Li Gel. (2019) Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food and Funct. 10(9):6121-6134.
- Fernandes A, Rodrigues PM, Pintado M, Tavaria FK. (2023) A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine. 115:154824.
- Liu Y, Su G, Zhou F, Zhang J, Zheng L, et al. (2018) Protective Effect of Bovine Elastin Peptides against Photoaging in Mice and Identification of Novel Antiphotoaging Peptides. J Agric Food Chem. 66(41):10760-10768.
- Kondo S. The roles of cytokines in photoaging. (2000) J Dermatol Sci. 23 Suppl 1:S30-6.
- Chung JH, Eun HC. (2007) Angiogenesis in skin aging and photoaging. J Dermatol. 34(9):593-600.
- Kim WS, Park BS, Sung JH. (2009) Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch Dermatol Res. 301(5):329-36.
- Li L, Ngo HTT, Hwang E, Wei X, Liu Y, et al. (2019) Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int J Mol Sci. 21(1):49.
- Malerich S, Berson D. (2014) Next generation cosmeceuticals: the latest in peptides, growth factors, cytokines, and stem cells. Dermatol Clin. 32(1):13-21.
- Fulop T, Khalil A, Larbi A. (2012) The role of elastin peptides in modulating the immune response in aging and age-related diseases. Pathol Biol (Paris). 60(1):28-33
- Lee SG, Ham S, Lee J, Jang Y, Suk J, et al. (2024) Evaluation of the anti-aging effects of Zinc-α2-glycoprotein peptide in clinical and in vitro study. Skin Res Technol. 30(3):e13609
- Asyi MS, Syahrizal D, Sary NL, Husna F. (2023) The impact of photoaging on skin: A systemic review analysis. J of Soc Res. 3(1):209-15
- Cai CS, He GJ, Xu FW. (2023) Advances in the Applications of Extracellular Vesicle for the Treatment of Skin Photoaging: A Comprehensive Review. Int J Nanomedicine. 18:6411-6423.
- Saklani R, Domb AJ. (2024) Peptide and Protein Stereocomplexes. ACS Omega. 9(16):17726-17740.
- Al-Mahmood SA, Abduljaleel AW, Abdul-Hussein L, Muneem AS. (2024) Oral Peptides and Protein Delivery. JMOB.1(1):01-06
- Slater G, Bachmid Z. (2023) Peptide Therapy Update. J Regn Bio Med. 5(6):322-4
- He B, Wang F, Qu L. (2023) Role of peptide–cell surface interactions in cosmetic peptide application. Front. Pharmacol. 14:1267765
- Mohtashami Z, Singh MK, Salimiaghdam N, Ozgul M, Kenney MC. (2022) MOTS-c, the Most Recent Mitochondrial Derived Peptide in Human Aging and Age-Related Diseases. Int J Mol Sci. 23(19):11991
- Bojarska J. (2021) Short Peptides: On the Trail of Future Stem Cell-Based Regenerative Therapies. Int J Nutr Sci. 6(1):1046.
- Jones L, Passegue E. (2021) STEM CELLS HOMEOSTASIS AND AGING. Innov Aging. 7(Suppl 1):259–60.
- Ferreira MS, Magalhães MC, Oliveira R, Sousa-Lobo JM, Almeida IF. (2021) Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules. 26(12): 3584
- Yang H, Zhang Q, Zhang B, Zhao Y, Wang N. (2023) Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs 21(3):144
- He X, Gao X, Guo Y, Xie W. (2024) Research Progress on Bioactive Factors against Skin Aging. Int. J. Mol. Sci. 25(7):3797
- Slivka JP, Bauer C, Younsi A, Wong MBF, Chan MKS, et al. (2024) Exploring the Molecular Tapestry: Organ-Specific Peptide and Protein Ultrafiltrates and Their Role in Therapeutics. Int Mol Sci.25(5):2863

