Stem Cell Therapy for Glioblastoma
Abby Lin and Vincent S Gallicchio*
Department of Biological Sciences College of Science Clemson University. Clemson, SC, USA, 29636
*Corresponding author: Vincent S Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA, 29636
Citation: Lin A, Gallicchio VS. (2023) Stem Cell Therapy for Glioblastoma. J Stem Cell Res. 4(1):1-12.
Received: March 28, 2023 | Published: April 19, 2023
Copyright© 2023 genesis pub by Lin A, et al. CC BY NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/JSCR.2023.4(1)-45
Abstract
Glioblastoma is a devastating grade IV cancer that affects the central nervous system and the spinal cord primarily in older adults but can also affect youth. The progression of the disease causes symptoms such as headaches and epilepsy. With such a severe and devasting prognosis and little ability to extend or cure the disease, researchers have been trying to find the best treatments to help patients suffering from Glioblastoma. Although the goal of Glioblastoma is not only to treat the disease but to cure it, current animal in vivo studies have shown promising therapeutic effects in mesenchymal stem cells, induced neural stem cells, and induced pluripotent stem cells. These stem cells have self-renewal capabilities, and stem cells, like the mesenchymal stem cells, can differentiate into various lineages from the mesoderm. While research has shown that each stem cell poses its own risk to the disease, their therapeutic effects have also shown encouraging and promising results. This review outlines the research, progress, and potential therapeutic effects different stem cells have on Glioblastoma. This study aimed to review the current progress researchers have made regarding stem cell therapeutic effects for Glioblastoma, how it is applied, and to discover the potential future progress and treatments these stem cells can create.
Keywords
Glioblastoma; Neural stem cells; Mesenchymal stem cells; Treatment; Tumor
List of Abbreviations
CD133+: heightened tumorgenicity, cRGD: Cyclic arginyl glycyl aspartic acid
CRISPR/Cas 9: Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 C6 cell: rat glioma cell line
DNA: deoxyribonucleic acid
EGFR: Epidermal growth factor receptor ESC: Embryonic stem cell
FADD: Fas-associated proteins with death domain GB: Glioblastoma
GBM8: Glioblastoma multiforme 8 GSC: Glioblastoma stem cell
iNSC: Human-induced neural stem cell HSC: Hematopoietic stem cell
HSPC: Hematopoietic and progenitor cell iNSC: Induced neural stem cell
iNSC-sTR: Induced neural stem cell-short tandem repeat iPSC: Induced pluripotent stem cells
MRI: Magnetic resonance imaging MSC: Mesenchymal stem cell NSC: Neural stem cell
PET: Positron emission tomography PTEN: Phosphatase and tensin homolog P13K: Phosphatidylinositol 3-kinase
scRNA-seq: Single-celled ribonucleic acid sequence
TD: Transdifferentiated
TGFβ: Transforming growth factor beta
TK: Thymidine kinase
TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand
Introduction
What is Glioblastoma?
Glioblastoma (GB) is considered a grade IV cancer of the central nervous system, according to the World Health Organization [1]. While secondary GBs can arise in children, primary GB is the most common, fatal brain tumor in adults [2, 3]. GB works aggressively, with the most common prognosis averaging 12-15 months [1]. While there are lower-grade gliomas where coincide chemotherapy is not always part of the treatment, the aggressive nature of the malignant gliomas obligates chemotherapy usage [4]. Malignant, or grade IV, gliomas are the invasive and lethal form with the shortest prognosis [4].
Along with the aggression of the disease, GB is also predisposed to necrosis, and the tumor is mitotically active [5]. Scientists debate whether GBs arise from a subculture of neural stem cells or from the transformation of differentiated astrocytes [6]. Further, recurrent tumors differ from the original tumor due to mutations and evolutions, limiting the information gathered from the initial biopsy when treating the recurred disease [7].
How do doctors differentiate between different glioblastomas? Gliomas often originate from three types of glial cells: oligodendrocytes, ependymal cells, and astrocytes - however, astrocytic gliomas hold 70% of all glioma origins [4]. The classification of each glioma is based on its cell origin and molecular characteristics, which includes acquired mutations [4].
Pathology of Glioblastoma
What drives GB and its progression? An epidermal growth factor receptor (EGFR) is a typical influencer, where phenotypic changes occur by over expression, amplification, and mutation [5]. Amplification of the EGFR happens through reverse transcription, which is then accompanied by EGFR over expression [5]. While the most common GB tumors reside in the frontal, temporal, and parietal lobes, there are rare GBs that can reside in the occipital lobe or the spinal cord [8]. A brainstem GB tumor is a rare form of GBs in adults that is not as rare in pediatric GBs [13]. Although it is not common, GB does not always stay local to the origin of the cancer - it can metastasize to other locations: the lungs, the lymph nodes, the bone, and the liver [8].
Changes in patients’ personalities and moods can occur due to GB; However, it can be mistaken as a psychogenic disorder or part of the “aging process,” which delays diagnosis - negatively affecting the prognosis [8]. Other symptoms of GB, such as epilepsy, headaches, intracranial pressure, nausea, and a slow neurocognitive function, are often experienced post-diagnosis [8] (Figure 1).
Figure 1: Neuroimages of glioblastoma in MRI, PET, and others [8].
Current Treatments for Glioblastoma
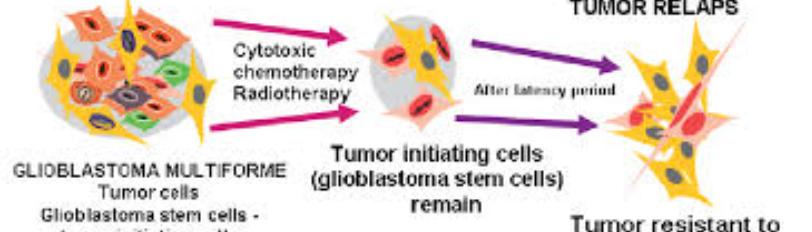
Different therapy options can increase the cancer's survival the cancer; however, they are rendered ineffective when it pertains to patients with grade IV GB [1]. Factors that cause the current therapy treatments to be weak are the blood-brain barrier, cancer stem cells, and the infiltration into the brain parenchyma [1]. Standard treatments, such as maximal safe surgical resection, followed by paired radiotherapy and chemotherapy with temozolomide (a first-line chemotherapy drug that causes apoptosis in GB), allow the average survival rate to stand between 14-16 months [7]. However, the surgical removal of the entire tumor mass is not feasible because the cells sit 2-3 cm from the original site, which could lead to a more aggressive regrowth post-removal [7].
While surgical resection is the current standard of care, it is case-specific based on the tumor’s size and shape, the location of blood vessels and arteries, and the sensitivity of the brain region [4]. Fortunately, patients with a 90% tumor resection have a one-year survival compared to patients that undergo less than 90% tumor resection [4]. Therefore, the vital ability to resect as much of the tumor mass as possible can significantly increase GB survival [4] (Figure 2).
Figure 2: Current therapy treatment approaches [13].
What are stem cells, and why are they being investigated?
Stem cells self-replicate, differentiate, and trope tumors, along with various other factors that could make them attractive therapeutic candidates for GB [5]. Further, they are pluripotent and give rise to various lineages of daughter cells [9]. For example, neural stem cells (NSCs) make cells differentiate into neurons, astrocytes, and oligodendrocytes [9]. NSCs reside in the subventricular zone and the hippocampus, giving them the preference to migrate to tumor masses [3]. These abilities are advantageous as they can simultaneously minimize the side effects of radiotherapy and chemotherapy while maximizing the drug delivery to the tumor site [3]. Due to stem cells’ ability to regenerate in the central nervous system after tissue injury from surgery or chemotherapy, scientists and doctors believe they can provide direct or indirect anti-tumor effects [5].
Stem cells are currently being studied as a standard of care for GB since they can be genetically modified to express various immunomodulatory substances to prevent tumor growth [3]. In addition, they are used for trafficking oncolytic viral vectors to fight against the tumor mass [3]. Another approach for stem cells is to combine them with prodrugs to convert them into an active form that will migrate toward the tumor [3].
Discussion
Glioblastoma stem cells (cancer stem cells)
Glioblastoma stem cells (GSCs) are found in human brain tumors [6]. GSCs were discovered to play essential roles in therapeutic resistance: radio resistance, chemoresistance, angiogenesis, invasion, and recurrence [6]. The resistance to radiotherapy and chemotherapy is potentially caused by a high capacity for extensive DNA repair, quiescence, higher mitochondrial reserve, and localization in the hypoxic niche [6]. GSCs are unstable mutationally, transcriptionally, and metabolically [10]. The instability causes these stem cells to metastasize and become unresectable able to adapt to new environmental factors [10]. While there are no indications about the tumor cell of origin for GSCs, it is inferred that the presence of GSCs could cause the malignant transformation of a normal tissue stem cell [6]. GSCs are not characterized by the presence or absence of particular molecular markers as they are not sensitive nor specific to a GSC population [6]. However, GSC characterization does not state that the activity of the stem cell is dormant but instead exists in different cellular versions of the tumor, which allows interconversion between GSC and non-GSC states [6]. Certain microenvironmental factors like nutrient deprivation, hypoxia, and radiation can change the dynamic of the regulation of interconversions and phenotypes like proliferation and quiescence [6].
Studies in GSCs
Primary tumor specimens' single-celled RNA sequences (scRNA-seq) have found intertumoral heterogeneity and a slow-divided quiescent population of stem-like cells in GB tumors [6]. For oligodendrogliomas, scRNA-seq has uncovered the presence of undifferentiated stem-like cells, indicating these cells promote tumor growth [6]. In a study with mutant astrocytomas and oligodendrogliomas, the stem cell and cell cycle genes had a positive correlation at the single-cell level [6]. Unlike NSCs which contain discrete self-renewing populations with the ability to multiply highly or cause inactivation [6].
Therapeutic stem cells
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are currently one of the most applied somatic stem cells in experimental therapies for regenerating damaged tissues [10]. MSCs are multipotent stem cells with self-renewal abilities and differentiate into osteoblasts, chondrocytes, and adipocytes - cell lineages of the mesoderm [10]. MSCs can be found and obtained from the bone marrow, adipose tissue, dental pulp, spleen, umbilical cord, etc. [10]. Experimental studies have shown that these stem cells have strong tumor tropism and can migrate to primary or metastatic cancers after infusion [10]. Their anti-tumor demonstrations exert anti-glioblastoma activity by controlling angiogenesis, regulating the cell cycle, and inducing apoptosis [10]. However, some experiments have found risks of tumor enhancement, cell proliferation, invasion, and aggressiveness after MSC therapy [10].
What are the anti-tumor effects of MSCs? They are considered the potential delivery of therapeutic agents for GB [5]. Tumor-associated MSCs are taken into the glioma’s microenvironment to promote tumor growth via cytokines, chemokines, and growth factors, acting as a trojan horse to deliver antitumor proteins, immune factors, long non-coding RNAs (or antitumor microRNAs), oncolytic viruses, or suicide genes as a result of tropism of the MSCs [5]. How does this induce tumor cell death? Cell death can occur by transducing MSCs with mRNA, which is then used to encode a pro-drug activating enzyme to function as a suicide protein [5]. The regression of the tumor occurs when the MSCs and suicide protein are injected into the tumor site [5]. This suicide protein changes non-toxic pro-drugs into toxic pro-drugs at the site, resulting in a synergistic effect to cause cell death [5]. It was found that phosphatase and tensin homolog engineered MSCs can also cause cytotoxic effects, and microRNAs can stimulate apoptosis or cell senescence when MSCs deliver them [5]. Consequences such as angiogenesis inhibition and apoptosis have decreased the GB tumor’s volume [10]. Still, the relation of whether it is due to the MSC source, the timing of experiments conducted, or the delivery route of the MSC is unknown [10].
What are the risks of MSC therapy? Could they pose a risk for tumor promotion? The short answer is yes, as the modulation, migration, and invasion of tumor cells have been found in MSC-conditioned media [5]. This conditioned media found the expression of six different types of proteins in the presence of C6 cells, which are closely related to cell differentiation and proliferation - indicating that MSCs may infiltrate the GB tumor but promote tumor growth at the secretion of exosomes [5]. MSCs that secrete chemokines can promote tumorigenesis via angiogenesis, proliferation, epithelial-mesenchymal transition, senescence, immune evasion, and metastasis [5] (Figure 3).
Figure 3: Main MSC mechanisms to control GB suppression (anti) or induction (pro) [10].
Neural stem cells
As discussed before, NSCs are closely associated with GB, giving them the potential to be optimal models for the exploration and knowledge of GB [5]. NSCs migrate approximately 50 minutes after transplantation, and the stem cell number increases slowly in the region, with significant expansion about two weeks after transplantation [3]. NSCs can self-renew and differentiate into neural progenitor cells of neuronal lineages, such as neurons, astrocytes, and oligodendrocytes [5]. Further, they are one of the most promising GB treatment strategies because they can naturally migrate to solid and diffuse GB deposits in response to chemotactic signals released by the cancer cells [12]. These progenitor cells are found in the brain and spinal cord, allowing them to easily differentiate into neural cells or glial progenitor cells [5]. The use of NSCs has revealed they are beneficial for treating GB due to their ability to deliver apoptosis-inducing ligands that target tumorigenic cells [5]. In preclinical models, NSC therapies have demonstrated potent anticancer efficacy, excellent tropism in areas of pathology, and a highly selective drug delivery directly into tumors [11].
Induced neural stem cells (iNSCs) undergo trans differentiation from a patient’s skin and can be used as drug carriers towards neural cells such as GB [5]. Their properties make them an attractive candidate for cancer therapy as they possess critical effective cell carrier systems such as a high expression of engineered transgenes, long-term survival in vivo, and no tumor formation [11]. Perhaps NSC-based cancer therapies could start by transdifferentiating skin fibroblasts into iNSCs that expand, engineer, and reimplant into GB patients [11]. Also, these derivatives are genetically engineered to express cytotoxic proteins to aid in cancer destruction since the cells naturally migrate toward the cancer site [5]. iNSCs carry therapeutic agents like thymidine kinase (TK), which phosphorylates the circulating ganciclovir into cytotoxic ganciclovir triphosphate to emphasize the method of action of the drug - the inhibition of DNA polymerase [5]. This mechanism kills the tumor cells and the iNSCs, too [5]. Another method is modifying NSCs with T-cells to create an innate reaction for engaging with the antigens of tumor cells [5]. Also, human iNSCs (h-iNSC) have tumor-homing capabilities with tropic tumor properties, allowing them to migrate to GB cells [12].
Studies of NSCs and iNSCs
NSCs were studied based on the tumor's location to observe their migration [5]. When NSCs were implanted just 2mm lateral from the tumor loci, NSCs were found to colocalize with many tumors but preferred to migrate to the tumor foci near the site where it was implanted [5]. However, NSCs have been found to increase tumor formation, like MSCs [5]. If a treated NSC used as a vector, or a trojan horse, becomes defective, the NSC can further the disease process [5]. In recent years, researchers have leaned towards using NSCs localized at the subventricular zone as the GB cell origin because immature outer subventricular zone glial cells share similar transcriptions to GB cells, indicating these cells may be GB precursors [5]. Further, PTEN mutations found in NSCs allow neoplastic transformation, unlike MSCs, suggesting the importance of knowing and understanding each cell of origin and its molecular features [5].
Bago and colleagues conducted two preclinical studies to observe the trans differentiation process to generate iNSCs for glioma treatment [5]. What is trans differentiation? It is the direct reprogramming of somatic cells into lineage-specific cells to become pluripotent stem cells [5]. In the first preclinical study, mouse and human iNSCs were modified to produce a transmembrane protein, tumor necrosis factor- related apoptosis-inducing ligand (TRAIL), which attracts Fas-associated proteins with death domain (FADD), to promote cell apoptosis and death in malignant cells [5]. The effectiveness was demonstrated by a decrease in the tumor size and an increase, or improvement, in glioma mice survival rates [5].
The second preclinical study tested a suicide-protein-based therapy [5]. Bago and his colleague’s gene- encoded an enzyme into the stem cell [5]. After injection, the enzyme can change the non-toxic prodrug into a toxic prodrug to regress the tumor cells, with the most common combination being the herpes simplex virus thymidine kinase with ganciclovir [5]. This combination converts ganciclovir into ganciclovir monophosphate, which then phosphorylates to ganciclovir triphosphate, a toxic antimetabolite that inhibits DNA polymerase to stimulate tumor cell death [5]. This second preclinical study demonstrated high therapeutic potential for GB treatment and profound antitumor efficacy [5].
Induced pluripotent stem cells
With modern-day technology, we can reprogram almost any somatic cell into a pluripotent embryonic stem cell-like state through the delivery into the somatic cells of a mixture of reprogramming transcription factors [5]. Induced pluripotent stem cells (iPSCs) proliferate infinitely in the culture and differentiate into ectoderm, mesoderm, and endoderm, therefore, able to develop all cells of an adult organism [5]. These iPSCs and their derivatives are helpful vehicle transport tools for GB stem cell therapy treatment [5].
Limitations to iPSCs
Like NSCs and MSCs, there are risks and limitations to iPSCs. These include tumorigenicity, immunogenicity, heterogeneity, and economic issues [5]. Further, iPSCs and iPSC-derived cells implanted in vivo have shown they can form cancerous teratomas, causing a stall in their current therapies [11]. However, these limitations must be addressed and removed to discover the full potential of iPSC technology as they have shown promising antitumor effect potential [5]. Gene editing technology has created iPSCs to observe cell line variation difficulties [5]. Researchers advise a plan to generate iPSC banks that consist of and cover most of the world’s population [5].
Other stem cells
Other stem cell shave also been investigated to observe their GB therapy effects. Embryonic stem cell (ESC) uses exosome delivery by modifying the complex with cRGD peptide for glioma therapy [5]. The results of these studies have shown ESC-exos possess great GB-targeting ability and significantly decrease the viability of cancer cells [5]. Studies have used stem cells to manipulate the microenvironment of embryos and discovered their ability to lessen the severity of malignancy because of the down regulation of the P13K pathway [5]. It was found that ESCs’ microenvironment could inhibit this pathway, promote apoptosis, and prohibit GB proliferation [5].
Results of hematopoietic stem cells (HSCs) showed high proliferation rates of GB cells when stimulated by TGF-β1 and interacting with HSCs [5]. HSC gene therapy was able to effectively transport TGFβ-blocking peptide to experimental GB with the combination of irradiation [5]. Therefore, the study demonstrated that TGFβ-blocking HSC gene therapy combined with irradiation resulted in a longer survival time than the control group [5]. Further, HSPC-derived cells depend on T-cells to differentiate into dendritic cells and activate T cells, promoting T-cell responses and glioma tumor rejection [5].
Results
Animal in vivo studies
Both in vitro and in vivo studies have been conducted to understand the subpopulations of tumor cells and their stem cell relations. It was discovered that some stem cells can initiate tumors or increase tumor heterogeneity after mice injection [6]. For somatic in vivo studies, CRISPR/Cas9 genomic editing experiments have been performed on GB mice models for general observation [6]. However, ethical and legal reasons require prospective isolation and propagation of GSCs methods regarding current technological advances [6].
Through animal in vivo studies, researchers have been able to molecularly engineer stem cells to prevent angiogenesis, deliver inflammatory cytokines, mediate immune response, initiate ligand-activated anti- tumor pathways, compete for specific pro-proliferate ligands to inhibit tumor growth, release anti-tumor toxins, include apoptosis, or cell suicide through enzyme pro-drug systems, deliver nanoparticles and oncogenic viral particles, and release vesicles containing anti-tumor microRNA [3]. For example, tracking the in vivo response of NSCs with GB revealed that the systemically administrated progenitor cells cross the barrier to localize in GB foci [3]. In a study by Tobias et al., a 46% increase in the median survival of mice treated with a combination of NSCs carrying oncolytic adenoviruses and radiation had the most remarkable efficacy reported in cases where stem cells were injected before chemoradiotherapy rather than after chemoradiotherapy [3]. Also, in vivo studies have shown that intravenously administered NSCs cross the blood-brain barrier to perform their functions without producing toxins in the brain [3].
Bago et al. conducted an in vivo study centered around iNSCs to observe the migration of iNSCs to GB in mice [11]. They recorded iNSCs’ ability to track GB cells that had invaded the parenchyma, with invasive CD133+ and GBM8 cells expressing mCherry being implanted in the frontal lobe of the mice [11]. After three days, iNSC-GFPFLs were injected at the site where GBM8 tumors were previously implanted [11]. After three weeks, mice were killed, and IHC was performed to discover the GBM8 and iNSC distribution; It was found that numerous GFP+ iNSCs had colocalized with the diffuse GBM cells [11].
In a following study, Bago et al. studied the efficacy of iNSC-sTR treatment on solitary human GB cells [11]. They discovered statistically significant reductions in tumor viability and an increased GB cell death when iNSC-sTR numbers increased [11] (Figure 3,4). Further, they determined iNSC-sTR treatment reduced tumor growth and decreased GB volumes by 123-fold after a month, and animals survived for more than two months before a recurrent tumor, whereas the control survived 28 days [11]. In an additional study to observe the efficacy of iNSC-sTR treatment on patient-derived human GB cells, results showed iNSC- sTR therapy had significant therapeutic effects against malignant and invasive GBs, which extended the survival of mice with the tumor [11]. In response to all their conducted studies, certain lines varied in their resistance and sensitivity to TRAIL but had significant cell death with iNSC-sTR at GB sites [11].
Figure 3: Efficacy of iNSC-sTR therapy [11].
Figure 4: In vivo migration of iNSCs to GBs [11].
Conclusion
Results of stem cell therapy
Current studies have shown stem cell ability to target brain pathologies. MSCs and NSCs were discovered to have high tropism to malignant gliomas because of overexpression in cell surface markers and the secretion of molecular signals in said tumor’s microenvironment [3]. As stated before, in vivo animal studies have demonstrated the modification of stem cells to target neoplasms directly, decrease tumor effects, and extend GB survival [3]. However, the adverse effects of specific stem cells, like NSCs, have shown that they can speed up tumor formation processes rather than inhibit them [5]. If an NSC used as a vector is defective, the stem cell could move further along the disease process [5]. Since NSCs are self- limited, the methodology used in treatment with these cells includes a termination process to limit the cells from progressing into the cell line [5].
What has been found?
Some researchers have found that GB might develop from NSCs, as NSCs are more prone to neoplastic conversion than differentiated cells [9]. This malignant change might occur due to DNA replication stress due to the transcription of incredibly long neural genes [9]. Also, cancer stem cells like GSCs act like tumor- initiating cells that promote tumor growth when inserted in the animal, but this does not mean it corresponds to being the cell of cancer origin [9].
What still needs to be studied?
It is vital to gain a clearer and deeper understanding of the molecular processes that drive CSC maintenance, plasticity, and resiliency to promote our ability to selectively target and reduce tumor- initiating and tumor-propagating populations [6]. Recently, there has been a clinical trial to test tumor- specific fluorescent staining to aid surgeons in differentiating between tumor and non-tumor cell tissue [4]. TD cells are preferred for cell replacement, as they avoid immune surveillance since it is a patient’s cells and can be used to create NSCs [12]. However, the efficacy of TD-derived human NSC cancer therapy still needs to be studied further [12].
Conclusion
Glioblastoma is a devastating disease with a concise survival rate. However, with current research, NSCs and iNSCs, MSCs, and iPSCs have promising effects in extending the prognosis. While the end goal is to treat and cure the disease, these are hopeful first steps to such advancements. Along with future advances in technology and procedures, stem cells have the potential to be the hero in GB treatment.
References
- Abadi B, Ahmadi-Zeidabadi M, Dini L, Vergallo C. (2021). Stem cell-based therapy treating glioblastoma multiforme. Hematol Oncol stem cell Ther. 14(1):1–15.
- Luo C, Song K, Wu S, Farrukh Hameed NU, Kudulaiti N, et al. (2021) The prognosis of glioblastoma: A large, multifactorial study. Br J Neurosurg. 35(5):555-61.
- Soomro SH, Ting LR, Qing YY, Ren M. (2017). Molecular biology of glioblastoma: Classification and mutational locations. J Pak Med Assoc. 67(9):1410–14.
- Benmelouka AY, Munir M, Sayed A, Attia MS, Ali MM, et al. (2021) Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int J Mol Sci. 22(5):2258.
- Carlsson SK, Brothers SP, Wahlestedt C. (2014) Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 6(11):1359–70.
- Attia N, Mashal M, Pemminati S, Omole A, Edmondson C,et al. (2021). Cell-Based Therapy for the Treatment of Glioblastoma: An Update from Preclinical to Clinical Studies. Cells, 11(1):116.
- Gimple RC, Bhargava S, Dixit D, Rich JN. (2019) Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 33(11-12):591–609.
- Wirsching HG, Galanis E, Weller M. (2016) Glioblastoma. Handb Clin Neurol. 134 : 381–97.
- Rodriguez SMB, Staicu GA, Sevastre AS, Baloi C, Ciubotaru V, et al. (2022) Glioblastoma Stem Cells-Useful Tools in the Battle against Cancer. Int J Mol sci. 23(9): 4602.
- Biserova K, Jakovlevs A, Uljanovs R, Strumfa I. (2021) Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells, 10(3):621.
- Nowak B, Rogujski P, Janowski M, Lukomska B, Andrzejewska A. (2021) Mesenchymal stem cells in glioblastoma therapy and progression: How one cell does it all. Biochim Biophys Acta Rev cancer. 1876(1):188582.
- Bagó JR, Alfonso-Pecchio A, Okolie O, Dumitru R, Rinkenbaugh A, et al. (2016) Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun. 7:10593.
- Bagó JR, Okolie O, Dumitru R, Ewend MG, Parker JS, et al. (2017) Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Tran Med. 9(375): eaah6510.

