Molecular Predictors of Prostate Carcinoma in Patients of Different Ages
Startsev V. Yu1*, Krivonosov D. I2 and Vorobyov S.L3
1,2St. Petersburg State Pediatric Medical University, Ministry of Health of the Russian Federation
3National Center for Clinical Morphological Diagnostics, St. Petersburg
*Corresponding Author: Startsev V. Yu, St. Petersburg State Pediatric Medical University, Ministry of Health of the Russian Federation.
Citation: Startsev V. Yu, Krivonosov D, Vorobyov S. L. Molecular predictors of prostate carcinoma in patients of different ages. J Can Ther Res. 4(1):1-10.
Received: November 14, 2024 | Published: November 29, 2024
Copyright© 2024 genesis pub by Startsev V. Yu, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/JCTR.2024.4(1)-37
Abstract
Introduction
The prevalence of the prostate cancer (PCa) among men in 105 countries ranks second. In the Russian Federation in 2006-2016, there was an annual increase in the absolute number of proven cases of PCa (+289.5%, or +90.6 per 100 000 male population). The average age of patients with diagnosed PCa in the world is 65 years, but since 1990, the incidence of cancer in men aged 20-40 years was characterized by an increase up to +2%/year (p<0.01). The possibilities of early diagnosis of this pathology in people under 50 years have not been studied enough.
Materials and methods
The study included 20 patients with verified PCa after radical surgery, distributed by age: under 50 years (group A, n=10) and over 50 years (group B, n=10). A retrospective analysis of medical documentation was carried out to assess age, anthropometric indicators, and levels of prostate-specific antigen (PSA).
Result
In group A, a patient with pT1cN0M0 ISUP-3 PCa, with PSA level of 9.86 ng/mL (maximum value) revealed the lowest (50%) E-cadherin level among patients in both groups. P53, EGFR, and TP 53 mutations were not detected. RB1 gene mutation (pT2aN0M0 ISUP-3, BMI=29.05 kg/m2, PSA=4.76 ng/ml) was detected in 1 (5%) patient younger than 50 years. Loss of PTEN gene heterozygosity was detected in 3 (15%) cases (2 in group A and 1 in group B); in these patients, an increase in PSA index (3.5-12.57 ng/ml) was noted, with an increase in age (39-61 years). Loss of PTEN heterozygosity was found in the youngest (39 years old) patient (the lowest PSA level=3.5 ng/ml and Ki-67<1%) and in the oldest (61 years old) patient (pT2cN0M1, PSA=12.57 ng/ml, Ki-67=5%). In patients of group A with PTEN deletion, the volume of E-cadherin tissue staining is 30% less than in patients of group B (70% to 100%).
Conclusion
According to data obtained on a small sample of patients, there is a possible association of PTEN gene mutation and E-cadherin staining level with the development of PCa before the age of 50, as well as with the development of clinically significant forms of that disease in older patients. PSA levels are higher in older patients, so this indicator should not be used as the only criterion for the presence of prostate cancer. Further study of the set of risk factors for the development of PCa will make it possible to adjust the diagnostic approach in young patients based on personal molecular genetic information.
Keywords
Prostate Cancer; Early diagnosis of prostate cancer; Immunohistochemical method of diagnosis; Prostate cancer at a young age
Introduction
Prostate cancer (PCa) ranks fifth in the global ranking of causes of male malignant neoplasm (MN) deaths [1]. According to the GLOBOCAN database, 1 414 259 new cases of prostate cancer and 375 304 deaths related to this disease were registered in 174 countries in 2020 [2]. An annual increase in proven cases of PCa in Russian Federation (RF) was proven: in 2006-2016 it amounted to 289.5%, or +90.6 per 100 thousand of male population [3]. Among all MN in men in the Russian Federation, PCa ranks second (14.3%) after trachea, bronchi and lung diseases (17.8%). In 2014, 37 186 new cases of prostate cancer were verified [4]. In men over 60 years, PCa occupies a leading position (18.5%) among the rest MN.
According to studies [5], between 1990 and 2020, the incidence of PCa at the age of 35-55 years at the time of diagnosis increased 3 significantly (from 2.3% to 9%), and the average age of PCa verification decreased from 72 to 68 years. The annual increase in the incidence of prostate carcinoma between the ages of 15 and 40 years worldwide is 2% (p < 0.01) [5]. One of the key factors that influenced the increase in the number of new cases of prostate cancer over the past 30 years was the introduction of testing of men's blood serum for prostate-specific antigen (PSA) [6]. According to [7], the incidence of disseminated PCa in young men turned out to be higher due to the fact that these people did not receive regular screening based on PSA levels [7].
Cancer screening tests are positioned as a tool for early diagnosis in order to increase life expectancy, but it is still unknown whether patient’s life expectancy will increase against the background of widely used screening tests. According to a systematic review and meta-analysis of randomized clinical trials [8], which included 2 111 958 people, with a median follow-up of 10 years, there was no significant difference in life expectancy for continuous screening of PCa with PSA testing (37 days; 95% CI, 37-73 days). This is an essential argument not in favor of a continuous blood PSA study to detect clinically significant PCa. The expediency of screening for PCa through blood testing for PSA has recently raised many questions. Thus [9], conducted a study proving that clinically significant forms of prostate cancer are detected in men with PSA level of 1.8–3 ng/ml [9].
This study included 6 006 men, with an average age of 55.9 years. In 11% of cases (n=670), PSA was 1.8-3 ng/mL (median PSA 2.1 ng/mL [IQR 1.9-2.5]) and in 6.3% of cases (n=377) - 3-10 ng/mL (median PSA 3.9 ng/mL [IQR 3.3-5.0]). All patients underwent magnetic resonance imaging (MRI) to assess the foci of tumor growth in the prostate (according to PI-RADS V2) and biopsy. PCa verified in 64 men (9.5%) in the group with low PSA: in 33 (51%) with Gleason sum (GS) = 6 (clinically insignificant PCa) and 31 (49%) with GS≥7. In men with high PSA levels, 61 (16%) were found to have PCa: 26 (42%) with GS=6; 35 (58%) - with GS≥7. A significant number of clinically significant forms 4 of PCa verified in men with low PSA levels (1.8–3 ng/ml), which additionally indicates the inferiority of testing. Aggravated heredity in the male line is considered to be an important factor in the early development of PCa, which predetermines an aggressive type of the course of the disease, with unfavorable outcome [10]. This fact indicates the need to examine men with a history of prostate cancer in the male line, with attention to genetic mutations BRCA2, ATM, etc., to identify groups at risk of early tumor dissemination. Consideration of somatic mutations allows early treatment to ensure a satisfactory life span.
New genetic variants of the development of PCa based on genes associated with PSA were identified [11]. At the first stage of the study, potential risk alleles were identified in men with prostate cancer and burdened heredity (491 cases with PCa + 429 control cases with benign prostatic hyperplasia, BPH). Multivariate analysis is based on PSA level, tumor size and stage, presence of metastases, GS, recurrence time and time to death since PSA test, and the expression of new (PABPC1, QK1, FAM114A1, MUC6, MYCBP2, RAPGEF4, RNASEH2B, ULK4, XPO7 and THAP3) and known PSA-associated genes (ATM, BRCA2, HOXB13, FAM111A, EMSY, HNF1B, KLK3, MSMB, PCAT1, PRSS3 and TERT). Many genes are associated with the risk of developing PCa in men with burdened heredity; the study shows the advantage of gene sequencing for selection of patients with a family history or an aggressive form of this disease [12]. (2016) presented data on the significant involvement of genes encoding enzymes of estrogen metabolism (CYP17, CYP19, COMT, CYP1B1 and UGT1A1) in the progression of prostate cancer [13]. Populations of men of African descent were determined, taking into account the multi-factorial development of prostate cancer and difference in the background of the studied groups (Democratic Republic of Congo: 162 patients with PCa and 144 with PH; Guadeloupe: 498 patients with PCa and 565 controls with PH). The relationship between the development of PCa and the expression of genes encoding androgen and estrogen metabolism was revealed [13].
Targeted PCa screening was conducted in men with BRCA1/2 mutations at 62 medical centers in 20 countries of the world. Men aged 40-69 years with BRCA1/2 germline mutations and a control group of men with burdened heredity but negative test for pathogenic BRCA1/2 mutation were included (n=2481: 791 BRCA1 carriers, 531 BRCA1 controls; 731 BRCA2 carriers, 428 BRCA2 controls). All participants underwent PSA testing: at PSA>3.0 ng/ml, a prostate biopsy was offered. Out of 199 (8%) cases with PSA levels>3.0ng/ml, 162 biopsies were performed, 59 cases of PCa were diagnosed (18 carriers + 10 BRCA1 controls; 24 carriers+7 BRCA2 controls). 66% of cases were identified in the groups of medium and high risk of developing PCa. For biopsies using PSA=3.0 ng/mL threshold with the presence of BRCA2, the prognostic value of a positive result (PPV) was 48%, i.e. twice as much as the PPV previously reported [14], indicate that long non-coding RNAs (lncRNA AC245100.4) are involved in the development and progression of PCa [15]. The expression of AC245100.4 was upregulated in PCa tissues and cell lines, and decreasing the concentration of RNA led to the suspension of tumor growth. An animal model shows that the proliferation and migration of atypical prostate tissue cells increases markedly with overexpression of AC245100.4. RNA blocking suppressed tumor cell proliferation and migration (dual analysis with oxidative enzyme luciferase and RNA immunoprecipitation assay results). This study opened up the scope for studying the characterization of tumor cell RNA.
In single domestic meta-analyses, information on the epidemiology and prevalence of prostate cancer in men of different ages over 26 years (from 1997 to 2021) was studied [17]. This paper describes the directions for the development of early diagnosis of clinically significant PCa using new molecular genetics and histological research methods that have not yet become widespread due to the high cost and small sample of young (under 50 years old) patients. Insufficient coverage in the world scientific literature of the issues of PCa verification in patients of working age does not yet allow us to speak confidently about the victory over this aggressive disease.
Objective: to determine the possibilities of early molecular diagnosis of PCa by immunohistochemical analysis of tumor tissues in patients of different ages.
Materials and methods: The medical documentation of 20 patients with PCa and, additionally, the histological structure of prostate slides after radical surgical treatment were studied: 10 patients under the age of 50 (group A, age 39-49 years; 42 ± 7.3 years) and 10 patients over 50 years old (group B, age 54-70 years; 62 ± 8.4 years). All patients underwent surgeries (laparoscopic and robot-assisted laparoscopic method) without complications of the immediate postoperative period (up to 30 days).
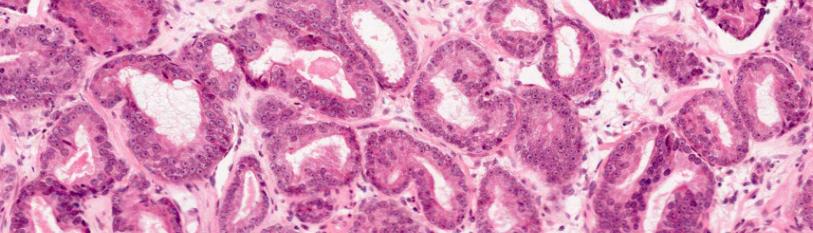
Ready-made histological coverglass preparations of prostate surgical material with verified acinar adenocarcinoma were used. In order to clarify the tumor characteristics and assess the background pathology, a standardized morphological study of additional coverglass preparations from paraffin blocks with the production of microtomic tissue sections with a thickness of 4 microns and staining with hematoxylin and eosin was carried out in accordance with the current WHO Classification (WHO Classification of Tumours Editorial Board. Urinary and male genital tumours Lyon (France): International Agency for Research on Cancer. 2022 [18]). The degree of differentiation of PCa on the Gleason scale (2012), the degree of spread (involvement of both lobes, gland apex, seminal vesicles), the presence of pericapsular, vascular or perineural invasion, and the assessment of resection margins were assessed. Immunohistochemical study was performed by the standard immunoperoxidase method (Roche Ventana BenchMark Ultra automatic immunohistostainer, USA; Leica BOND RX, Germany) on paraffin sections using monoclonal antibodies to β-catenin (clone β-catenin-1, Dako), to bcl-2 (clone bcl-2 1100105, Leica), to E-cadherin (clone 36, Ventana), to p53 (clone DO-7, Dako), Ki67 (clone MIB-1, Dako). A molecular-genetic study of the presence of PTEN, RB1, TP53, and EGFR gene deletions was also performed. All studies were conducted at the National Center for Clinical Morphological Diagnostics (St. Petersburg, Russia).
The Stastica for Windows software (version 12) was used to perform statistical analysis on the clinical data obtained. Quantitative parameters (such as age, BMI, PSA levels, Ki-67, P53, β-catenin, E-cadherin, BCL-2, EGFR, TP53, RB1, and PTEN) and qualitative parameters (such as malignancy criteria, tumor stage, postoperative material charactesristics at the resection margin, and involvement of specific structures) were compared between patient groups using the Mann-Whitney and Wald tests due to the small sample size. The frequency characteristics of qualitative indicators (e.g., staining and mutations) were evaluated using nonparametric methods, including the chi- square (χ²) test, Yates-corrected chi-square, and Fisher’s exact test.
Results
The body mass index (BMI) of group A patients ranged from 23.1 to 31.48 (27.29 ± 4.19), of which 80% of patients were overweight with an average BMI of 27.51, 10% were obese (BMI 31.48) and 10% were of normal body weight (BMI 23.1) (Table 1). In group B, the BMI index ranged from 24.22 to 33.9 (29.06 ± 4.84): excessive weight was noticed in 60% of patients (25.31–29.74), obese - in 20% of patients (BMI 30 and 33.9), and 20% were with normal anthropometric indicators (BMI 24.22 and 24.81).
|
Indicator |
Values by group |
P |
||
|
Criteria |
A (< 50 y.o.), n=10 |
B (> 50 y.o.), n=10 |
||
|
Age (year) |
M ± s.d |
42 ± 7 |
67.56 ± 12.44 |
<0.05 |
|
min / max |
35 / 49 |
54 / 70 |
||
|
Me (LQ; UQ) |
44.5 (41;46) |
62 (58;67.5) |
||
|
BMI (kg/m2) |
M ± s.d |
27.29 ± 4.19 |
29.06 ± 4.84 |
>0.05 |
|
min / max |
23.1 / 31.48 |
24.22 / 33.9 |
||
|
Me (LQ; UQ) |
27.47 (26.43;28.41) |
25.9 (25.41;28.88) |
||
|
PSA (ng/ml) |
M ± s.d |
6.34 ± 2.82 |
13.23 ± 5.84 |
<0.05 |
|
min / max |
3.5 / 9.86 |
7.4 / 19.08 |
||
|
Me (LQ; UQ) |
6.33 (4.86;8.67) |
11.01 (9.25;13.64) |
||
|
Malignancy category |
ISUP*-1 |
4 |
4 |
|
|
ISUP-2 |
3 |
4 |
||
|
ISUP-3 |
3 |
1 |
||
|
ISUP-4 |
- |
1 |
||
|
MN stages |
pT1cN0M0 |
3 |
|
|
|
pT2N0M0 |
|
4 |
||
|
pT2aN0M0 |
4 |
|
||
|
pT2bN0M0 |
|
1 |
||
|
pT2cN0M0 |
3 |
1 |
||
|
pT3aN0M0 |
|
2 |
||
|
pT3bN0M0 |
|
1 |
||
|
pT2cN0M1 |
|
1 |
||
Table 1: Characteristics of the patients, enrolled in the present study.
International Society of Urological Pathology
Preoperative PSA levels in group A ranged from 3.5 to 9.86 ng/ml (6.34 ± 2.82) and in group B - from 7.4 to 19.08 ng/ml (13.23 ± 5.84) (Table 1). Distribution by stages of PCa in group A: 3-pT1cN0M0, 4-pT2aN0M0, 3-pT2cN0M0, in group B: 4-pT2N0M0, 1-pT2bN0M0, 1-pT2cN0M0, 2-pT3aN0M0, 1-pT3bN0M0, 1-pT2cN0M1.
The average age in groups A and B at the time of diagnosis differed by 19 years (43.9 vs. 62.9, P<0.05), the age difference in the youngest and oldest patients was 35 years (35 years in group A and 70 years in group B), the minimum difference amounted to 5 years (49 years in group A and 54 years in group B). Age indicators by group are shown in (Figure 1).
Figure 1: Age indicators in patients, enrolled in the present study.
The average BMI was higher in group A, but the maximum value was 2.42 kg/m2 higher in group B patients (P>0.05), as it is shown in (Figure 2).
Figure 2: BMI indicators in patients of the studied groups.
The PSA level at the time of diagnosis in group B patients was significantly higher than in group A patients (13.23 ± 5.84 vs. 6.34 ± 2.82, P<0.05), the difference averaged 6.89 ng/ml (Figure 3).
Figure 3: Comparison of PSA levels in patients of the studied groups.
When reviewing slides by a third-party morphologist, all ISUP criteria were confirmed: a tumor in the apex of the gland was absent in the samples of group A.In 40% of cases (4 specimens) and in 30% of cases in group B (3 specimens). Both lobes of the gland are present in all areas of prostate tissue, without perineural, lymphovascular invasion and urethral lesions. The most common and highly malignant prostate tissues were in older patients (group B). There were no patients with prostate cancer in stages T3a, T3b-T4 in group A, and there were also no cases with ISUP>3.
Molecular genetic analysis of the specimens revealed: in group A, the degree of Ki-67 staining was detected on average in 3% of tissues (0.9 - 5%) while in group B, it was detected in 6% of tissues (2 - 12%; P<0.05) (Figure 4).
Figure 4: Characterization of material staining when using tissue marker Ki-67 in the studied specimens.
In patients of both groups, the level of β-catenin in the tissue was 3 points, with 100% membrane staining. The distribution of E-cadherin by the degree of staining intensity in points in groups A and B was on average 2 (from 1 to 3 points) and an increase in staining intensity by 71.4% (on average 2.8 points, P<0.05), respectively. As a percentage, the amount of E-cadherin in group B samples also exceeded the indicators of group A by 13% (100 to 87%). A decrease in the amount of E-cadherin was noted in group A patients with the following tumor characteristics: 2 patients with T1cN0M0 (ISUP 3 and 2), 1 – T2aN0M0 (ISUP-2) and 1 – T2cN0M0 (ISUP-2), the smallest T1c tumor verified during biopsy, with an increase in PSA to 9.86 ng/ml (the highest PSA threshold in group A), had the highest degree of cell malignancy (ISUP-3) and the lowest volume of E-cadherin (50%) among all patients.
The accumulation of P53 in the materials of both groups did not differ, amounting to 1% in each sample. But in group A, 3 tissue samples with non-cluster staining were observed. EGFR and TP53 mutations were not detected. RB1 gene mutation was detected in a 44-year-old patient (group A), with BMI=29.05, PSA=4.76 ng/mL, with PCa stage T2aN0M0, ISUP-3. Loss of heterozygosity of the PTEN gene was detected in 3 cases (2 in group A and 1 in group B).
Anthropometric data, PSA level, degree of malignancy and tumor spread, presence of changes according to the results of molecular genetic analysis in the studied patients are presented in Table 2.
Table 2: Characteristics of the patients with established loss of heterozygosity of the PTEN gen.
Discussion
There are no both conventional algorithms and sequences for screening PCa based on serum PSA levels, not the age range of people who are need to be screened. There is a risk of overdiagnosis of clinically insignificant tumor (e.g., in cT1aN0M0 ISUP-1 stage) in the preclinical diagnosis of this MN [19], which may have a negative effect on the patient's quality of life. In the world literature, the search for individual criteria, in addition to the PSA index, for early diagnosis of PCa continues. There is a widespread opinion about the relationship of hereditary factors of PCa with the environmental quality index (EQI), with a decrease in which the risk of developing cancer increases. The study [15], showed that the incidence of PCa is directly related to poor environmental quality (CI 16.28-48.91), which indirectly indicates a potential increase in the incidence of that neoplasm with a decrease in EQI [16].
Changes in biological processes, hormone excretion, inflammation, DNA damage and suppression or overexpression of genes are the result of a complex environmental impact on the human body. An important conclusion of the study is that not only mutational load analysis, but also correlation of environmental factors that can be a promoter of the development of this tumor should be used to predict the behavior of PCa [16]. In the world literature, older male’s age is still considered to be the relevant age for the development of PCa. Following the COVID-19 epidemic, which required extraordinary solutions from epidemiologists and infectious disease specialists, the wave of development of MN of different localizations, including PCa, among people of working age perplexes immunologists and oncologists [20, 21]. After two decades of decline, the age-standardized mortality rate for MN increased over 2019-2021 for all cancers (HR=1.6%, 95% CI 0.6%-2.6%), especially for PCa (HR=5.1%, 95% CI 2.2%-8.2%) [22]. The issues of early detection of aggressive PCa in people of working age are of particular importance. However, in the available medical literature, we could not find information about conducting IHC tests of prostate tissue samples in patients with PCa younger than 50 years old.
In our study, there is no relationship between BMI and age and loss of heterozygosity of the PTEN gene. When analyzing statistical data, it was found that the PSA level increases markedly (from 3.5 to 12.57) with the age of patients (39-61 years), which may indicate that it is inappropriate to focus solely on the PSA level when screening for prostate cancer in young men.
The level of E-cadherin in tumor tissue in young patients (group A) is 30% lower than in older patients (70% to 100%). The youngest (39 years old) of the studied patients had a deletion of the PTEN gene and had the lowest levels of PSA (3.5 ng/ml) and Ki-67 in tumor tissue (<1%). In the oldest (61 years old, prostate cancer in the pT2cN0M1 stage) patient with loss of PTEN heterozygosity, all indicators were inversely related: PSA =12.57 ng/ml, Ki-67=5%. Thus, there is a possible correlation between the loss of heterozygosity of the PTEN gene and the level of E-cadherin staining with the development of prostate cancer at a young age (up to 50 years old), as well as with the development of more aggressive forms of carcinoma in older people (over 50 years old), up to its dissemination. However, the study sample should be increased to obtain an evidentiary conclusion.
Conclusions
According to the data obtained on a small sample of patients with PCa (n=20), a possible relationship of PTEN gene mutation and E-cadherin staining level with the development of that disease in young men, as well as with the verification of clinically significant forms of tumor in patients over 55 years of age was noted. PSA levels were higher in age-matched patients (group B), so it is inappropriate to focus solely on this indicator when diagnosing young men.
Further study of the set of risk factors for the development of PCa will make it possible to adjust the diagnostic approach in younger’s based on personal molecular genetic information. That was our pioneer study, and in the aim of obtaining more convincing evidence, it is necessary to increase the sample of the patients and control groups in the future.
References
- Sadeghi-Gandomani HR, Yousefi MS, Rahimi S, Yousefi SM, Karimi-Rozveh A et al. (2017) The Incidence, risk factors, and knowledge about the prostate cancer through Worldwide and Iran. WCRJ. 4(4):e972.
- Wang L, Lu B, He M, Wang Y, Wang Z, et al. (2019) Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019 Frontiers in Public Health.
- Aksel EM, Matveev VB. (2019) Statistics of malignant neoplasms of the urinary and male genital organs in Russia and the countries of the former USSR. Oncourology. 15(2):15-24
- Kaprin AD, Starinsky VV, Petrova GV. (2014) Malignant neoplasms in Russia in 2014 (incidence and mortality). Moscow: FSBI "Moscow Scientific and Research Oncological Institute named after P.A. Herzen" Ministry of Health of Russia, 2016:250.
- Bleyer A, Spreafico F, Barr R. (2020) Prostate cancer in young men: An emerging young adult and older adolescent challenge. Cancer. 126(1):46-57.
- Kramer BS, Brown ML, Prorok PC, Potosky AL, Gohagan JK. (1993) Prostate cancer screening: what we know and what we need to know. Ann Intern Med. 119(9):914-23.
- Sartor O. (2020) Why is prostate cancer incidence rising in young men? Cancer. 126(1):17-18.
- Bretthauer M, Wieszczy P, Løberg M, Kaminski MF, Werner TF et al. (2023) Estimated Lifetime Gained With Cancer Screening Tests: A Meta-Analysis of Randomized Clinical Trials. JAMA Intern Med. 183(11):1196-203.
- Möller F, Månsson M, Wallström J, Hellström M, Hugosson J, Godtman R.A. (2024) Prostate Cancers in the Prostate-specific Antigen Interval of 1.8–3 ng/ml: Results from the Göteborg-2 Prostate Cancer Screening Trial. European Urology.2024,ISSN 0302-2838.
- Nicolosi P, Ledet E, Yang S, Michalski S, Freschi B, et al. (2019) Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 5(4):523-28.
- Schaid DJ, McDonnell SK, FitzGerald LM, DeRycke L, Fogarty Z et al. (2021) Two-stage Study of Familial Prostate Cancer by Whole-exome Sequencing and Custom Capture Identifies 10 Novel Genes Associated with the Risk of Prostate Cancer. Eur Urol. 79(3):353-61.
- Brureau L, Moningo D, Emeville E, Ferdinand S, Punga A, et al. (2016) Polymorphisms of Estrogen Metabolism-Related Genes and Prostate Cancer Risk in Two Populations of African Ancestry. PLoS One. 11(4):e0153609.
- Bancroft EK, Page EC, Castro E, Lilja H, Vickers A et al. (IMPACT Collaborators; Moss S, Eeles RA. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 66(3):489-99.
- Xie H, Zhao J, Wan J, Zhao J, Wang Q, et al. (2021) Long non‑coding RNA AC245100.4 promotes prostate cancer tumorigenesis via the microRNA‑145‑5p/RBBP5 axis. Oncol Rep. 45(2):619-29.
- Jagai JS, Messer LC, Rappazzo KM, Gray CL, Grabich SC, Lobdell DT. (2017) County-level cumulative environmental quality associated with cancer incidence. Cancer. 123(15):2901-08.
- Startsev VYU, Shpot EV, Karaev DK, Krivonosov DI. (2022) Detection of prostate cancer in young and middle-aged men. Bulletin of Urology. 10(1):110-20.
- WHO classification of tumours series, 5th ed.; vol. 8. Available from: https://tumourclassification.iarc.who.int/chapters/36
- Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, et al. (2002) Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 94(13):981-90.
- Shin BNH, Tan S, Rhee H, Chung E. (2024) Impact of the COVID-19 pandemic on delivery of prostate cancer care in Australia: An interrupted time series analysis. Int J Cancer. 154(6):1003-1010.
- Star J, Bandi P, Siegel RL, Han X. (2023) Cancer Screening in the United States During the Second Year of the COVID-19 Pandemic. J Clin Oncol. 41:4352-9.
- Fedeli U, Barbiellini AC, Han X, Jemal A. (2024) Changes in cancer-related mortality during the COVID-19 pandemic in the United States. J Natl Cancer Inst. 116:167-9.

