Mayada F Karkar1, Mohamed E Elkomy2, Ghada Mohammed2, Mahmoud Nageh2, Oday Adel2, Amira Mohsen2 and Hamdi Hamama2
1Faculty of Dentistry, Mansoura University, Egypt
2Faculty of Dentistry, New-Mansoura University, Egypt
*Corresponding author: Hamdi Hosni Hamdan Hamama, Clinical Associate Professor, Conservative Dentistry Department, Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
Citation: Karkar FM, Elkomy ME, Mohammed G, Hamama H, Adel O, et al. Micromorphological and Elemental Analysis of Dentin Following Application of Silver Diamine Fluoride (SDF) Products. J Oral Med and Dent Res. 4(2):1-10.
Received: December 07, 2023 | Published: December 22, 2023.
Copyright© 2023 genesis pub by Karkar MF, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: http://doi.org/10.52793/JOMDR.2023.4(2)-49
Abstract
Silver diamine fluoride products (SDF) are used to arrest dental caries in deep carious lesions as (SDF) is considered as effective and easy to apply. Twenty-four premolars were collected; enamel layer is removed then divided randomly into 3 groups. Group 1 (control) was left without treatment of any product. Group 2 was treated with pure SDF. Group 3 was treated with SDF+KI. Three specimens were observed with scanning microscope and energy dispersive x-ray analysis (EDX).
Keywords
Silver diamine fluoride; Potassium iodide; Energy dispersive x-ray analysis; Dentin; Dental caries.
Introduction
Dental caries is a multifactorial ‘biofilm-mediated’ disease led to destruction of hard tooth substrate. This multifactorial, oral disease is caused primarily by an imbalance of the oral flora (biofilm) due to the presence of fermentable dietary carbohydrates on the tooth surface over time. Enamel and dentine develop cavities in distinct ways. Caries in dentine refer to both mineral demineralization and organic matrix degradation of the type I collagen fiber network, whereas caries in enamel refer to the dissolution of highly mineralized tissue because of an attack by bacterial acid [1]. Dental caries, despite being a mostly unavoidable condition, continues to pose an important issue for elderly and geriatric patients' oral health. It has a major adverse effect on people's quality of life and functional limitations, particularly on underprivileged people and communities [2]. Currently, Fluoride products are widely used, which has significantly improved children's oral health and reduced the prevalence of dental cavities. Preschoolers still have many untreated caries lesions compared to other age groups, and the decay component of the dexterity index is the greatest in this group. This can be attributed to limited financial resources, limited access to basic dental care, and greater costs for restorative dentistry, untreated dental caries have an adverse effect on the general health, social well-being, and academic performance of children from low-income nations [3].
The use of (SDF) for arresting dental caries was firstly tried in Japan.in the 1960s [4]. The pioneering human clinical trial that has shown preventive effect on caries lesions was a split-mouth study in the permanent lower first molars of 25 children aged 6 to 8. SDF treatments exhibited 73% reduction of developing new lesions compared to the control groups. The first two such trials focused on caries arrest in young children was after three decades, but also evaluated the incidence of new lesions as a secondary outcome. Silver diamine fluoride (SDF) (Ag (NH3)2F) is a colorless solution with antimicrobial and demineralizing properties. SDF is commonly available as a 38% solution containing silver and fluoride ions. Silver showed potent antimicrobial activity on the oral biofilm. also prevents the degradation of dentine collagen through the inhibition of proteolytic peptidases in dentine and saliva. [5] SDF therapy is aligned with current WHO goals and in line with the United States Institute of Medicine's criteria. In 2020, the British Society of Pediatric Dentistry published its support of the use of SDF to treat caries. [6] In 2021, the World Health Organization included SDF as an useful health system medicament that complies with both adults and children demands.
The United States Food and Drug Administration (FDA) has cleared SDF as a Class II medical device for professional use to manage dentine hypersensitivity. In 2017, Canada Health approved SDF as a dental treatment. SDF is cleared as an anti-hypersensitivity agent in the United States [4]. SDF induces tertiary dentin formation at 6 weeks after treatment when performed in healthy pulp conditions. It can be used in people who are not a good fit for conventional restorative treatment and young children who are too young to receive conventional restorative treatment in a dental chair. It can be used to treat older adults who have limited access to dental clinics, as well as people with special needs who are unable to cooperate with dental treatment [8]. The disadvantage of SDF is discoloration of teeth that has limited the use of SDF. Hence, the application of potassium iodide (KI) following SDF has been proposed to reduce the discoloration [9]. Some dentists think that KI applying following SDF might affect bond between the tooth surface and restorative materials. However, others said that SDF and KI combination has no significant effect on teeth bonding with restorative materials. KI is favorable to provide better aesthetic appearance [10,11]. Clinical tries found that SDF may cause transient gingival irritation requiring no treatment [12]. A systematic review concluded that SDF causes mild, reversible pulpal inflammation and is generally biocompatible [13]. The null hypothesis is that there is no significant difference between SDF and SDF+Ki in micromorphology and elemental analysis.
Materials and Methods
The study proposal was reviewed and approved by Ethical Committee of Faculty of Dentistry, Mansoura University, Egypt.
Exclusion Criteria
Presence of caries, Presence of restorations, Presence of pit and fissure sealant and severely extracted teeth.
Specimen Preparation
For micromorphological analysis Specimens were collected of sound freshly extracted premolars preserved in distilled water , embedded in self-cure acryl and cut with PICCO155 precision cutter to remove the enamel from their occlusal surfaces transversally and expose the dentine , put them in glass jars filled with distilled water then cleaned with ultrasonic cleaner for 3 minutes to remove the smear layer, after drying them surfaces for better detection of the surfaces details, divided them into groups a control group which remained with no additives , SDF and SDF+ KI ware applied to the others groups following manufacturer instructions. SDF is applied on dentin surface using micro brush. KI is applied on the third group following SDF application using micro brush until forming creamy white surface. The teeth were put in a SPI coater to coat them with gold to be able to see their details under the electronic microscope.
Micromorphology and Elemental Observation
Scanning electron microscope (JEOL) is used for magnification of 16, 500, 1000 and 2000 for each tooth to examine their dentinal surfaces and the precipitates formed on them. Four micrographs were captured for each specimen in different magnifications. For elemental analysis the same steps were made for specimens’ preparation. X-max with Oxford software to determine the amount of the following minerals: silver (Ag), calcium (Ca), Phosphorus (P) and fluorine (F). measurements were expressed as a relative percentage weight of identified elements. The change of each usual mineral amount and the new minerals deposited was measured, then the gold of the coating was removed from the elements.
Results
SEM Observation Analysis
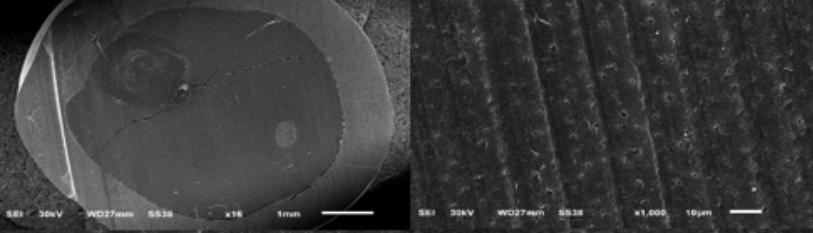
Micro-morphological analysis was observed under scanning electron microscopy (SEM) which showed [figure 1] patent dentinal tubules of (group 1) untreated specimens (Control specimens).
Figure 1: Cross-sectional SEM images of (group 1) untreated with SDF products showing patent and rough dentinal tubules, using different magnifications.
The Second group which was treated with SDF (Toothmate) on the dentin surface under (SEM) has shown blocked dentinal tubules, increased size of hydroxyapatite crystals and needle shape crystals following Silver Diamine Fluoride application which was remarkable in this study (Figure 2).
Figure 2: Cross-sectional SEM images of (group 2) treated with SDF (Toothmate) showing blocked dentinal tubules, large size needle crystals, using different magnifications.
Micro-morphological analysis, the third group treated with (Riva Star) SDF following KI under (SEM) has shown occluded dentinal tubules and large size amorphous polygonal shape crystals of SDF+KI precipitation.
Figure 3: Cross-Sectional SEM images of (group 3) treated with SDF+KI (RivaStar) showing blocked dentinal tubules, large size Polygonal crystals of its precipitation, using different magnifications.
EDX Analysis
Energy dispersive X-ray spectrometry (EDX) has examined the elemental analysis of each group and the results illustrated that SDF applied group showed largest rate of minerals silver, phosphorus, fluoride and calcium rather than other samples. [Figure 4 a, b, c]
Figure 4a: Elementally under Energy dispersive X-ray spectrometry (EDX), the specimen displayed O with (80.80% weight) and (90.62% Atomic), P with (5.91% weight) and (3.42% Atomic), and Ca with (13.30% weight) and (5.95% Atomic).
Figure 4b: Elementally, under (EDX), the specimen displayed F with (34.28% weight) and (57.43% Atomic), Na with (2.74% weight) and (3.79% Atomic), P with (7.90% weight) and (8.11% Atomic), Ca with (28.86% weight) and (22.92% Atomic), and Ag with (26.23% weight) and (7.74% Atomic).
Figure 4c: The specimen’s 3rd spectrum K with (19.898% weight) and (37.25% Atomic), Ca with (13.15% weight) and (24.02% Atomic), Ag with (1.09% weight) and (0.74% Atomic), and I with (65.87% weight) and (38.00% Atomic).
Discussion
The present study investigated the micromorphological analysis of SDF and SDF/KI on dentin surface. To knowledge, this is the first study to use (Tooth mate SDF). The choice of Scanning electron microscope could be attributed to its ability to reveal crystal shape of each product precisely. Elemental analysis of this study was observed with energy dispersive X-ray spectroscopy within the scanning electron microscope as it provides accurate mineral percentage with high spatial resolution and provides atomically resolved elemental maps. (14) Silver diamine fluoride related to an interaction with hydroxyapatite crystals of tooth surface to form calcium fluoride (CaF2) and silver phosphate (Ag3PO4). These have been thought to prevent dental caries, re-mineralize tooth surface and form crystals. Chemical reaction was simplified:
Ca10(PO4)6(OH)2 + Ag(NH3)2F CaF2 + Ag3PO4 + NH4OH
CaF2 has cubic crystals shape, Ag3PO4 has globular and needle shape crystals. (15). Silver phosphate is an unstable and reduced into metallic silver by reducing agents like light exposure, showing different crystals shape. (16-18) Metallic silver and all silver compounds contribute to protect dentin surface and form black discoloration. Concerning fluoride reactions, it can react with hydroxyapatite crystals in many ways to form Fluro-hydroxyapatite and calcium fluoride [19]. Calcium fluoride is important because it reacts as a reservoir of fluoride ions at low PH and promoting remineralization [20]. Fluorapatite is more stable than hydroxyapatite in acidic conditions which make tooth more resistant to caries [22-25]. Silver diamine fluoride reacts with dentin collagen as silver ions is electron acceptors which has low affinity and large atomic radius. These ions make a bond with sulfur and nitrogen within histidine and cysteine of dentin collagen to form protective layer of silver-protein complex on dentin surface [26].
The result indicated that the application of SDF products increased the mineral/organic content and the crystallite size of the hydroxyapatite crystals in the treated dentin specimens. This might be attributed to the presence of mineral crystals which act as crystal growth centers. Silver diamine fluoride is an alkaline solution which is favorable condition to form fluorapatite crystals. Collagen structure act as scaffold for crystal growth [27]. This study associated these compositional and microstructural alterations, as well as the formation of different crystalline phases on the dentin surfaces. Elemental analysis of third group showed high amount of Iodine which can be attributed to formation of Agi compounds [28, 29]. Silver content was less in SDF/KI group than SDF group, this might reduce the antimicrobial effect of SDF. Application of KI immediately after SDF is a way to reduce the discoloration produced by SDF. Iodine reacts with excess silver ions to form a yellowish layer of silver iodine which defense against wearing effect [30]. Elemental analysis of third group revealed that application of KI following SDF reduces Calcium content, this might reduce demineralizing effect of SDF.
The outcomes of elemental analysis agreed with Staxrud F and Gadallah LK studies, as they showed higher content of silver in SDF group than SDF/KI group. While it disagrees with Lee K study that showed no difference between the two products [8, 31, 32]. The hypothesis of micromorphological analysis agrees with Cifuentes-JimÉNez CC which showed more occluding dentinal tubules in SDF/KI group than SDF group [2]. the null hypotheses is rejected after observations, results and finding. One of limitation of this study (in vitro application) is the intraoral conditions (e.g.: saliva, plaque PH values and microbial composition). However, this study has important advantage regarding high level of scientific control.
Conclusion
It was concluded that the application of SDF showed less occluded dentinal tubules than application of KI following SDF on dentin surface. SDF group showed needle crystals, while SDF/KI showed amorphas crystals. Elemental analysis of SDF group showed a higher amount of silver than SDF/KI so it might have higher antimicrobial effect. The application of KI after SDF forms a thin layer of Agi which resists erosion. SDF may be a good choice for treating dental caries as it has high antimicrobial effect. While SDF/KI may be favorable for treating dental erosion.
References
1. Pitts NB, Twetman S, Fisher J, Marsh PD. (2021) Understanding dental caries as a non-communicable disease. Br Dent J. 231(12):749-53.
2. Cifuentes-JimÉNez CC, BolaÑOs-Carmona MV, Enrich-Essvein T, GonzÁLez-LÓPez S, ÁLvarez-Lloret P. (2023) Evaluation of the remineralizing capacity of silver diamine fluoride on demineralized dentin under pH-cycling conditions. J Appl Oral Sci. 31:e20220306.
3. Corrêa-Faria P, Viana KA, Raggio DP, Hosey MT, Costa LR. (2020) Recommended procedures for the management of early childhood caries lesions - a scoping review by the Children Experiencing Dental Anxiety: Collaboration on Research and Education (CEDACORE). BMC Oral Health. 20(1):75.
4. Zheng FM, Yan IG, Duangthip D, Gao SS, Lo ECM, et al. (2022) Silver diamine fluoride therapy for dental care. Jap Dent Sci Rev. 58:249-57.
5. Dias FA, Vidal CMP, Comnick CL, Xie XJ, Berger SB. (2023) Effect of silver nanoparticles associated with fluoride on the progression of root dentin caries in vitro. Plus one.18(1):e0277275.
6. Horst J, Heima M. (2019) Prevention of Dental Caries by Silver Diamine Fluoride. Compend Contin Educ Dent. 40:158-63.
7. Manuschai J, Talungchit S, Naorungroj S. (2021) Penetration of Silver Diamine Fluoride in Deep Carious Lesions of Human Permanent Teeth: An In Vitro Study. Int Jour Dent. 2021:3059129.
8. Gadallah LK, Safwat EM, Saleh RS, Azab SM, Azab MM. (2023) Effect of silver diamine fluoride/potassium iodide treatment on the prevention of dental erosion in primary teeth: an in vitro study. BDJ Open. 9(1):24.
9. Haiat A, Ngo HC, Samaranayake LP, Fakhruddin KS. (2021) The effect of the combined use of silver diamine fluoride and potassium iodide in disrupting the plaque biofilm microbiome and alleviating tooth discoloration: A systematic review. Plus one. 16(6):e0252734.
10. Turton B, Horn R, Durward C. (2020) Caries arrest and lesion appearance using two different silver fluoride therapies with and without potassium iodide: 6-month results. Heliyon. 6(7):e04287.
11. Magno MB, Silva LPd, Ferreira DM, Barja-Fidalgo F, Fonseca-Gonçalves A. (2019) Aesthetic perception, acceptability and satisfaction in the treatment of caries lesions with silver diamine fluoride: A scoping review. Int Jou Paed Dent. 29(3):257-66.
12. Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, et al. (2005) Efficacy of Silver Diamine Fluoride for Caries Reduction in Primary Teeth and First Permanent Molars of Schoolchildren: 36-month Clinical Trial. Jour Den Res. 84(8):721-4.
13. Zaeneldin A, Yu OY, Chu C-H. (2022) Effect of silver diamine fluoride on vital dental pulp: A systematic review. Jour Dent. 119:104066.
14. Slater TJ, Lewis EA, Haigh SJ. (2016) Energy Dispersive X-ray Tomography for 3D Elemental Mapping of Individual Nanoparticles. J Vis Exp. 113:52815.
15. Lou YL, Botelho MG, Darvell BW. (2011) Reaction of silver diamine fluoride with hydroxyapatite and protein. Jour Dent. 39(9):612-8.
16. Amirulsyafiee A, Khan MM, Harunsani MH. (2022) Ag3PO4 and Ag3PO4–based visible light active photocatalysts: Recent progress, synthesis, and photocatalytic applications. Catalysis Communications. 172:106556.
17. Andrés M, Vela P, Jovaní V, Pascual E. (2019) Most needle-shaped calcium pyrophosphate crystals lack birefringence. Rheumatology. 58(6):1095-8.
18. Dong P, Yin Y, Xu N, Guan R, Hou G, et al. (2014) Facile synthesis of tetrahedral Ag3PO4 mesocrystals and its enhanced photocatalytic activity. Materials Research Bulletin. 60:682-9.
19. Ogard B, Seppä L, Rølla G. Professional topical fluoride applications-clinical efficacy and mechanism of action. Adv Dent Res. 8(2):190-201.
20. Mei ML, Chu CH, Low KH, Che CM, Lo EC. (2013) Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 18(6):e824-31.
21. Zhao IS, Mei ML, Burrow MF, Lo EC, Chu C-H. (2017) Effect of Silver Diamine Fluoride and Potassium Iodide Treatment on Secondary Caries Prevention and Tooth Discolouration in Cervical Glass Ionomer Cement Restoration. Int Jour Mol Sci. 18(2):340.
22. Okazaki M, Miake Y, Tohda H, Yanagisawa T, Matsumoto T, et al. (1999) Functionally graded fluoridated apatites. Biomaterials.20(15):1421-6.
23. Okazaki M. (1992) Heterogeneous synthesis of fluoridated hydroxyapatites. Biomaterials. 13(11):749-54.
24. Okazaki M, Miake Y, Tohda H, Yanagisawa T, Takahashi J. (1999) Fluoridated apatite synthesized using a multi-step fluoride supply system. Biomaterials. 20(14):1303-7.
25. Roche KJ, Stanton KT. (2014) Measurement of fluoride substitution in precipitated fluorhydroxyapatite nanoparticles. Journal of Fluorine Chemistry. 161:102-9.
26. Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries "silver-fluoride bullet". J Dent Res. 88(2):116-25.
27. Firouzmandi M, Vasei F, Giti R, Sadeghi H. (2020) Effect of silver diamine fluoride and proanthocyanidin on resistance of carious dentin to acid challenges. Plos one. 15(9):e0238590.
28. Sayed M, Tsuda Y, Matin K, Abdou A, Martin K, et al. (2020) Effects of mechanical abrasion challenge on sound and demineralized dentin surfaces treated with SDF. Scientific Reports. 10(1):19884.
29. Detsomboonrat P, Thongmak P, Lertpayab P, Aiemsri W, Sooampon S. (2022) Optimal concentration of potassium iodide to reduce the black staining of silver diamine fluoride. Journal of Dental Sciences. 17(1):300-7.
30. Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, et al. (2005) An in vitro model to measure the effect of a silver fluoride and potassium iodide treatment on the permeability of demineralized dentine to Streptococcus mutans. Aust Dent J. 50(4):242-5.
31. Staxrud F, Becher R, Syverud M, Azulay N, Valen H. (2023) Silver release from dentine treated with combinations of silver diamine fluoride, potassium iodide and etching. Biomater Investig Dent. 10(1):2191634.
32. Lee K, Ahn J, Kim JS, Han M, Lee J, et al.(2021) Effect of Sodium Fluoride Varnish and Potassium Iodide on Remineralization Efficacy of Silver Diamine Fluoride. J Korean Acad Pediatr Dent. 48(4):467-75.