Prevention of Human Papillomavirus Associated Cancer
Shayoli Sarkar1, Siddheesh Rajpurohit2, Sheetal Chauhan3 and Ajit Singh4*
1Department of Pharmacy Practice, Samskruti College of Pharmacy, Jawaharlal Nehru Technological University, Hyderabad, India
2Department of Pharmacy Practice, Manipal college of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India
3Department of Pharmacology, Melaka Manipal Medical College, Manipal Academy of Higher Education, Manipal, India
4National Director for Research, World Youth Heart Federation, India; Co-founder & CEO, CliMed Research Solutions, India
*Corresponding author: Ajit Singh, National Director for Research, World Youth Heart Federation, India; Co-founder & CEO, CliMed Research Solutions, India.
Citation: Sarkar S, Rajpurohit S, Chauhan S, Singh A. (2021) Prevention of Human Papillomavirus Associated Cancer. J Can Ther Res. 1(1):1-5.
Received: Aug 5, 2021 | Published: Aug 27, 2021
Copyright© 2021 genesis pub by Sarkar S, et al. CC BY NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Abstract
Abstract
HPV (Human papillomavirus) infects epithelial cells, and their replication cycle is intimately linked to epithelial differentiation. A subset of mucosal-infective HPVs, also known as ‘high risk’ (HR) HPVs, causes cervical disease and could be classified as low or high grade. The HR-HPVs related infection can affect at any stage of life, or several individuals may experience during their lifetime. Our robust immune system plays a crucial role in recognizing and clearing the virus, but sometimes we need potent therapy to eliminate or prevent it. This review deals with the prevention of HPV from attacking host cells, and the methods may include vaccination or acute treatment. The short overview also enlightens the pathogenesis, mode of infection, and evidence-based prevention strategies of the HPV and associated cancer.
Keywords
Genital cancer; Keratinocyte; Viral gene expression; Sexual transmission
Introduction
Human papillomavirus (HPV) causes epithelial differentiation. There are around 200 plus different HPV genotypes that are identified to date, and each one of them displays strict tissue specificity for infection. Human papillomavirus infection can result in a range of benign lesions, like verrucas on the feet, common warts on the hands, or genital warts. HPV infects the multiplying basal epithelial cells where its dsDNA enters the nuclei. According to the World Health Organization (WHO), approximately 561,200 new cancer cases (around 5.2% of all new cancers) are due to HPV infection. Upon basal cell division, an infected daughter cell begins the process of keratinocyte differentiation that triggers a tightly orchestrated pattern of viral gene expression to accomplish a productive infection [1]. A subset of mucosal-infective HPVs, also known as ‘High Risk’ (HR) HPVs, causes cervical disease, categorized as low or high grade [2].
Human papillomavirus is a type of viral infection that tends to transmit from skin to skin contact, through sexual contact, and they can affect genitals, mouth, or throat. Many papillomaviruses are known to reduce the risk of immune clearance by causing chronic asymptomatic infections accompanied by long-term virion production with only limited viral gene expression [3]. According to the Centre for Disease Control and Prevention (CDC), HPV is the most common Sexually Transmitted Infection (STI). It’s so common that most sexually active people will get some variety of it at a certain point in time, even if they have few sexual partners. Some cases of genital HPV infection may not result in any health problems [4,5]. In the majority of cases, infected cells are eliminated by the immune system. Occasionally, elimination fails, and HPV infection becomes chronic. Replication of HPVs in multiplying epithelial cells is due to the increased expression of the E6 and E7 oncoproteins. These oncoproteins are responsible for the genomic instability, disruption of the cell cycle, cell proliferation, immortalization, and malignant transformation of HPV-infected cells [6]. The infection with certain HPV types also results in a certain variety of cancers like the anus, vulva, vagina, penis, and oropharynx, which are preventable by using similar primary prevention strategies like those for cervical cancer. Cancer continuance is due to persistent infection with an HR-HPV [7]. HR-HPV disease is responsible for more than 99.7% cervical cancers in women, and a subset of oropharyngeal cancers, predominantly in men. HPV16 (HR-HPV genotype-16) is the most prevalent worldwide and the primary cause of HPV-associated cancers. The objective of this review paper is to highlight the prevention of this virus to cause cancer and how it invades the host body [8].
Biology of HPV
Two products of HPV, i.e., E6 and E7, contribute to the pathogenesis of cancer [9]. HPV virus tends to get integrated with the DNA of the host nucleus further E6 combine with p53 (responsible for arresting the cell cycle in the G1 phase), which leads to degradation of p53 activity. Control over the cell cycle is lost when E7 binds to the cyclin-dependent kinase inhibitor [10].
Mode of Infection
HPV commonly spread through sexual contact, but transmission through non-sexual contact (through fomites) has been known to occur [11]. There are several risk factors which can contribute to HPV infection like early onset of sexual activity, multiple sexual partners, and use of oral contraceptives (more than ten years). Smoking and low social-economic status have reported increasing the risk of HPV infection in individuals. Usually, the virus is cleared by the action of the immune system, but oncogenesis is linked to the persistence of infection [12].
Pathogenesis
The HPV infects the squamous epithelial cells, which have a tendency to proliferate and quickly get access to the basal cell during injury or trauma. In basal cells, HPV induces the expression of genes (viral) that help in the viral replication. This interaction of host cells and HPV occurs via heparin sulfate proteoglycans and alpha-6-integrins. For initiation of the replication, the early proteins, i.e., E1 and E2, are responsible. The E2 is the transcriptional repressor of the E6 and E7 that controls the expression of E6 and E7. The mechanism of replication is a rolling circle in which the viral genome gets integrated with the human genome. This integration disturbs the E2, which results in the higher expression of both E6 and E7 and leads to cell transformation. After the replication of the virus, L1 and L2 gene are responsible for the formation of the capsid of the virus, and the virus gets released with the help of E4 protein [13-15].
Evidence-Based HPV Prevention
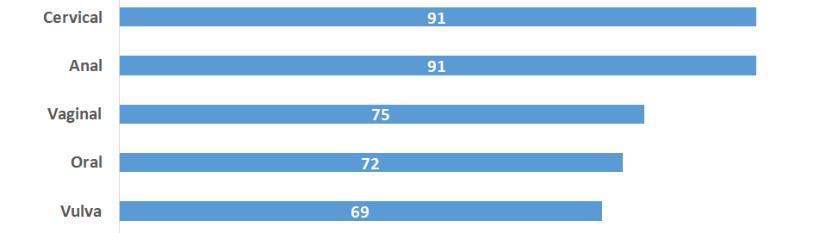
The easiest way to prevent HPV is to practice safe sex. Bivalent and quadrivalent vaccines for HPV are available for use. Both of them are directed toward the HPV 16 and HPV 18 type of HPV. Quadrivalent vaccination is also effective against HPV 6 and HPV 11, which are responsible for the anogenital wart. So far, these vaccines are available to prevent cervical cancer, but nowadays, it is useful to prevent a large number of cancer cases, which include the vulva, vaginal, penis, anus, oropharynx cancer [Figure 1] [16,17].
Figure 1: Cancer linked to HPV infection.
These vaccines are available for prophylactic use or treatment bsssut not for cure or treatment and also do not prevent the progression of the disease. The two HPV vaccines, i.e., Cervarix and Gardasil, are approved for the use in cervical prevention by FDA [18]. Gardasil is now approved to be used as a preventive treatment for genital warts and HPV associated lesions of the anogenital region. Advisory Committee on Immunization Practice suggests that girls and boys must be routinely vaccinated at the age of 11or 12 with two doses of vaccination with six months gap. People with the age group of 15 to 26 are also esssligible to have the HPV vaccination with a three-dose schedule. This committee also suggests that bisexual, gay men be vaccinated by the age of 26. People above age 45 are now also eligible for the vaccination [17,19,20].
Strategies to Prevent HPV
Vaccination coverage is very important to reduce the load of the cancer burden associated with the HPV. Strategies must be developed to promote the HPV vaccination, which includes the recall or reminder system, standing order for vaccination, education to the community, to provide more vaccination sites. Schools and colleges can be a target as HPV vaccination sites as it will cover most of the eligible age groups [21].
Conclusion
HPV is the most common sexually transmitted infection, mainly causes cervical cancer and genital warts. The quadrivalent HPV vaccine is 99% effective at preventing the high-grade cervical lesions caused by HPV types 16 and 18 that are precursors to cervical cancer and is equally effective at preventing HPV 6 and 11-related genital warts when given to HPV-naive individuals. It can be given to patients prior to HPV exposure. The debate is currently ongoing with regard to whether the vaccine will become a part of the mandatory vaccination schedule. The burden and cost of HPV-associated disease and cancer remain an important public health problem. Reducing the burden of HPV-associated cancer and disease through vaccination requires an integrated approach that includes clinical medicine, public health, and public policy.
Financial Support or Sponsorship
Nil.
Conflict of Interest
Authors do not declare any conflict of interest related to this study.
References
- Harden ME, Munger K. (2017) Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 772:3-12.
- Graham SV. (2017) The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci (Lond).) 131(17):2201-21.
- Vonsky M, Shabaeva M, Runov A, Lebedeva N, Chowdhury S, et al. (2019) Carcinogenesis Associated with Human Papillomavirus Infection. Mechanisms and Potential for Immunotherapy. Biochemistry (Mosc). 84(7):782-99.
- Alhamlan FS, Al-Qahtani AA, Al-Ahdal MN. (2015) Current studies on human papillomavirus in Saudi Arabia. J Infect Dev Ctries. 9(6):571-76.
- Egawa N, Egawa K, Griffin H, Doorbar J. (2015) Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 7(7):3863-90.
- Hakim AA, Lin PS, Wilczynski S, Nguyen K, Lynes B, et al. (2007) Indications and efficacy of the human papillomavirus vaccine. Curr Treat Options Oncol. 8(6):393-401.
- Gellin B, Modlin JF, Barr E, Tamms G. (2007) Quadrivalent human papillomavirus vaccine. Clin Infect Dis. 45(5):609-17.
- Skeate JG, Woodham AW, Einstein MH, Da Silva DM, Kast WM. (2016) Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum Vaccin Immunother. 12(6):1418-29.
- Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, et al. (2012) EUROGIN 2011 roadmap on prevention and treatment of HPV related disease. Int J Cancer. 131:1969-82.
- Arbyn M, De Sanjose S, Saraiya M, Sideri M, Palefsky J, et al. (2012) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta analysis. Lancet Infect Dis. 131(9):1969-82.
- Mayeaux Jr EJ. (2008) Reducing the economic burden of HPV-related diseases. Journal of the American Osteopathic Association. 108(4 Supplement 2):S2.
- Scully C. (2002) Oral squamous cell carcinoma; from a hypothesis about a virus, to concern about possible sexual transmission. Oral Oncol. 38:227-34
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX. (2002) Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 21:1510-07.
- Evander M, Frazer IH, Payne E, Qi YM, Hengst K, et al. (1997) Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J Virol. 71:2449-56
- Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, et al. (1999) The L1 major capsid protein of human papillomavirus type 11 recombinant virus like particles interacts with heparin and cell surface glycosaminoglycans on human keratinocytes. J Biol Chem. 274:5810-22.
- Arulponni TR, Janaki MG, Nirmala S, Ramesh BS, Rishi KS, et al. (2010) Carcinoma cervix treated with radiotherapy-our experience with emphasis on our concern. J Obstet Gynecol. 60:61-05
- Armstrong EP. (2010) Prophylaxis of cervical cancer and related cervical disease: A review of the cost effectiveness of vaccination against oncogenic HPV types. J Manag Care Pharm. 16:217-30.
- Braaten KP, Laufer MR. (2008) Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Am J Obstet Gynecol. 1(1):2.
- Bloem P, Ogbuanu I. (2017) Vaccination to prevent human papillomavirus infections: From promise to practice. PLoS Med. 14(6):e1002325.
- Villa LL. (2007) Overview of the clinical development and results of a quadrivalent HPV (types 6, 11, 16, 18) vaccine. Int J Infect Dis. 11:S17-25.
- Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. (2010) Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 102(5):325-39.

