Electromyographic Study for Swallowing Muscles in Normal and TMD Patients
Faisal Taiyebali Zardi, V. Nagalaxmi, Brajesh Gupta, Ayesha Sadathullah*, Rishika Reddy and Mohammed Haris Iqbal
Sri Sai College of Dental Surgery, Hyderabad, India
*Corresponding author: Ayesha Sadathullah, Sri Sai College of Dental Surgery, Hyderabad, India.
Citation: Zardi FT, Nagalaxmi V, Gupta B, Sadathullah A, Reddy R, Iqbal MH. Electromyographic study for swallowing muscles in normal and TMD patients. J Oral Med and Dent Res. 6(1):1-13.
Received: September 25, 2024 | Published: January 02, 2025
Copyright© 2025 genesis pub by Zardi FT. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/JOMDR.2025.6(1)-80
Abstract
Background
Temporomandibular disorder (TMD) is a multifactorial disease often linked to parafunctional habits such as clenching and grinding. These habits are known to influence the activity of facial muscles, potentially leading to conditions like obstructive sleep apnea (OSA) and its variant, dysphagic OSA.
Methods
This study evaluated the activity of four facial muscles (temporalis, masseter, digastric, and sternocleidomastoid) in 30 TMD patients and 12 healthy individuals using a 4-channel surface electromyography (sEMG) with Biopak software. Muscle activities were measured during rest, clenching, and swallowing. Data were analyzed statistically using the Mann-Whitney U test with a significance level set at p ≤ 0.05.
Results
The study found hyperactivation of all four muscles in TMD patients compared to healthy controls.
The temporalis muscle showed significant hyperactivation on both sides during rest (p=0.001 and p=0.000), clenching (p=0.000 and p=0.001), and swallowing (p=0.001 and p=0.000). Similar significant results were observed for the masseter, digastric, and sternocleidomastoid muscles. These findings suggest an increased masticatory muscle activity in TMD patients, potentially contributing to parafunctional habits and dysphagic OSA.
Conclusions
This study underscores the importance of early detection and multidisciplinary management of TMD to address parafunctional habits and prevent associated conditions like dysphagic OSA. The use of 4-channel sEMG provides valuable insights into the neuromuscular aspects of TMD, guiding clinicians towards improved diagnostic and therapeutic strategies.
Keywords
Temporomandibular Joint; Temporomandibular Joint Disorders; Obstructive Sleep Apnea; Dysphagic Obstructive Sleep Apnea; Facial Muscles; Surface EMG; Rest; Clenching; Swallowing.
Clinical Relevance Statement
The present study highlights the increased activity of facial muscles (temporalis, masseter, digastric, and sternocleidomastoid) in TMD patients during rest, clenching, and swallowing which was measured using a 4-channel surface electromyography. This hyperactivity is linked to parafunctional habits and may contribute to conditions like dysphagic OSA. Understanding these neuromuscular dynamics advances our knowledge of TMD and OSA pathogenesis and underscores the need for multidisciplinary approaches in diagnosis and treatment, aiming to improve clinical outcomes and quality of life for TMD sleep disorder patients. Clinicians are encouraged to consider these findings for early intervention and comprehensive management strategies.
Introduction
Temporomandibular disorder (TMD) is a multifactorial disease which is associated with multiple perpetuating, predisposing, and initiating factors [1-2]. It is most commonly encountered disorder among woman when compared to men [3]. This is probably correlated to increased level of production of estrogen hormone during the 2nd to 4th decades of life which ultimately declines during the phase of menopause [4-5]. One of the early sign of temporomandibular joint abnormality is presence of sounds during function [6]. The prevalence of TMD is commonly observed between the age group of 20 to 40 years [7].
Temporomandibular disease is a range of conditions which is commonly characterized by heterogeneous signs and symptoms and has been reported to be the 2nd most common musculoskeletal or neuromuscular disorders and is often associated to headache [8,10].
TMDs are considered as the primary cause of non-odontogenic pain in the orofacial region. The temporomandibular joint (TMJ) may be affected by inflammatory, traumatic, infectious, congenital, developmental, and neoplastic disease as seen in other joints [11]. Temporomandibular disease generates pain that is often unilateral in nature and referred to the ears, temporal and periorbital regions, the angle of mandible and to the posterior aspect of the neck [12,14].
Several authors hypothesized that TMD is often associated with obstructive sleep apnea (OSA [15-16]. The presence of OSA may aggravate oral parafunctional habits during sleep [17]. Studies further suggest an association between TMD and oral parafunctional habits. Clenching or grinding are often correlated to TMD [18]. The parafunctional habits are correlated to TMD pathogenesis [19].
The most common parafunctional habit evaluated is clenching [20]. Few studies have evaluated the correlation of TMD with an important and perpetuating function that occurs in daytime and during sleep i.e., swallowing. It is well known fact that the swallowing pattern differs among the population and that an alteration in positioning the tongue generates difference in both of maxillary growth and displacement of teeth [21,23]. Swallowing takes less than a second [24,26]. during which the activation of muscles induces contact between the teeth and controls stability of jaw, hyoid bone and TMJ [27].
Stomatognathic system activities, especially speaking, breathing, eating, and swallowing, are often negatively impacted by impairments of the temporomandibular joint and masticatory muscles [28]. Diagnostic tests for TMD include Jaw tracker, Joint vibration analysis, T-scan system, and electromyography.
The study of electrical activity of muscle is called electromyography (EMG). The instrument that was used to measure the electrical activity of muscles is called an electromyogram. An electromyograph is a pattern of muscular electrical activity that has been recorded. The first studies of dental applications of electromyography were published in the 1952 [29]. EMG was as used as diagnostic tool in 1996 by Okeson for evaluation of muscles [30].
EMG has three types of electrodes available. Surface electrodes are the ones which record the signals that are been generated on the surface of the skin, but these electrodes cannot detect deep potential signals occurring within the cell. Needle electrodes are the one that are inserted deep into the tissue, which records the action potential from the muscles and microelectrodes are the one which records the action potential the nerves [29].
Surface Electromyography (sEMG) is a technique in which electrodes are placed on the skin overlying a muscle to detect the electrical activity of the muscle. It’s a non-invasive and safe procedure which is indicated to study the muscle activity, relative change in timing of the muscle, assess motor control and coordination, evaluate mastication and post-surgical muscle function [29].
The aim of the present study is to evaluate oro-facial facial muscles during rest, clenching and swallowing in TMD patients using 4 channel surface EMG with Biopak software.
Methodology
Patient selection
30 TMD patients and 12 normal patients between the age range of 20 to 30 years, with a mean age of 27.4 years (Table:1) were selected from X college of dental surgery, INDIA.
|
AGE |
||||
|
Case/controls |
Gender |
Mean |
N |
Std. Deviation |
|
Cases |
Male |
29.4 |
10 |
7.486 |
|
Female |
28.32 |
20 |
7.499 |
|
|
Controls |
Female |
26.5 |
12 |
4.011 |
Table 1: Demographic data of age and gender among cases and controls
Patients’ selection criteria were as follows: Angle’s Class I Molar relation (i.e., normal intermaxillary dental relationship), good symmetry of dental arches, and no refractive errors. Individuals with dental braces, congenital oral cavity deformities, systemic or oral diseases, missing teeth, prosthetic rehabilitation, piercings, neurological conditions, a history of mental health issues, physiotherapy, subjects taking drugs other than nonsteroid inflammatory drugs, paracetamol, or minor opioid analgesics were excluded.
TMD patients were enrolled in the study. Examination was performed using a standardized form in which included history and duration of the diseases, palpation at rest, in maximal voluntary contraction i.e., clenching and during mandibular motions of the masticatory and neck muscles, palpation of TMJ, assessment of spontaneous and triggered pain using a visual analog scale, as suggested by Okeson [30].
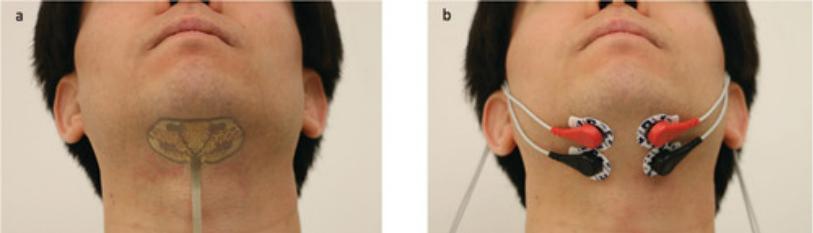
Before the evaluation, all the males were well-shaved. To place the electrodes, the subjects were asked to sit in an upright position with occlusal plane parallel to the floor. Skin was disinfected using spirit and cotton. To position the electrodes, subjects were requested to close their mouths and clench where the bulging of the muscle served as a templet in placement of the electrode. Ground electrodes were placed on the right and left trapezius muscle bilaterally which aids in conduction of signals. Bilateral sEMG electrodes were placed on temporalis muscles, masseteric muscles, digastric muscles, and sternocleidomastoid muscles (Figure 1a and 1b).
Figure 1a and 1b: Bilateral sEMG electrodes were placed on temporalis muscles, masseteric muscles, digastric muscles, and sternocleidomastoid muscles.
The instrument used was directly interfaced with a computer, which presented the data graphically. The signals were averaging over 25 milliseconds, with muscle activity of the 4 tested muscles expressed in microvolts. The sEMG test was performed on each patient while at rest, clenching and swallowing (Figure 2).
Figure 2: sEMG test was performed on each patient while at rest, clenching and swallowing.
Data Evaluation and Statistical Analysis
The parameters evaluated for both TMD patients and normal cases were muscles tension (mV) and muscles on right and left side in percentage of balancing (%) were evaluated. Data were evaluated on statistical ‘‘GraphPad’’ software. A normality test was performed in order to assess the data distributions. The intergroup differences were assessed using a nonparametric test (Mann–Whitney U test) in the group that passed the normality test. In TMD patients to correlate the balancing and frequency of muscles, Statistical significance was set at 0.05.
Results
In the present study the distribution of subjects was as 30 TMD patients (10 males and 20 females) and 12 healthy individuals. The demographic age distribution among TMD patients being 29.40 (±7.4) years in males and 28.32(±) years in females.
TMD patients had a higher activation of temporalis with p-value 0.001 on the right side and 0.000 on the left side during rest. A higher activation of temporalis with p-value 0.000 on the right side and 0.001 on the left side during clenching, and a higher activation of temporalis with p-value being 0.001 on the right side and 0.000 on the left side during swallowing. The mean value showed a statistically significant difference between cases and controls (Table 2).
|
|
Cases/Controls |
N |
Mean Rank |
Sum of Ranks |
P value |
|
Temporails Right Side Clench |
Cases |
30 |
22.62 |
656 |
0.000* |
|
Controls |
12 |
17.08 |
205 |
||
|
Temporails Left Side Clench |
Cases |
30 |
22.5 |
652.5 |
0.001* |
|
Controls |
12 |
17.38 |
208.5 |
||
|
Temporails Right Side Swallow |
Cases |
30 |
24.95 |
723.5 |
0.001* |
|
Controls |
12 |
11.46 |
137.5 |
||
|
Temporails Left side Swallow |
Cases |
30 |
25.59 |
742 |
0.000* |
|
Controls |
12 |
9.92 |
119 |
||
|
Temporails Right Side Resting |
Cases |
30 |
25 |
725 |
0.001* |
|
Controls |
12 |
11.33 |
136 |
||
|
Temporails Left side Resting |
Cases |
30 |
25.24 |
732 |
0.000* |
|
Controls |
12 |
10.75 |
129 |
Table 2: Mann Whitney Test Comparing Temporalis Emg At Clench Resting And Swallow Between Cases and Controls.
A higher activation of masseter with p-value 0.000 on the right side and 0.000 on the left side during rest. A higher activation of masseter with p-value 0.00 on the right side and 0.05 on the left side during clenching, and a higher activation of masseter with p-value being 0.000 on the right side and 0.05 on the left side during swallowing. The mean value showed a statistically significant difference between cases and controls (Table 3).
|
|
CASES/CONTROLS |
N |
Mean Rank |
Sum of Ranks |
P value |
|
Masseter Right Side Clench |
Cases |
30 |
21.52 |
624 |
0.00* |
|
Controls |
12 |
19.75 |
237 |
||
|
Masseter Left Side Clench |
Cases |
30 |
22.83 |
662 |
0.05* |
|
Controls |
12 |
16.58 |
199 |
||
|
Masseter Right Right-Side Clench |
Cases |
30 |
22.88 |
663.5 |
0.00* |
|
Controls |
12 |
16.46 |
197.5 |
||
|
Masseter Left Right Side Clench |
Cases |
30 |
23.34 |
677 |
0.05* |
|
Controls |
12 |
15.33 |
184 |
||
|
Masseter Right Side Resting |
Cases |
30 |
25.48 |
739 |
0.00* |
|
Controls |
12 |
10.17 |
122 |
||
|
Masseter Left Side Resting |
Cases |
30 |
25.47 |
738.5 |
0.00* |
|
Controls |
12 |
10.21 |
122.5 |
Table 3: Mann Whitney Test Comparing Masseter Emg At Clench Resting And Swallow Between Cases And Controls.
A higher activation of digastric muscle with p-value 0.000 on the right side and 0.000 on the left side during rest. A higher activation of digastric with p-value 0.001 on the right side and 0.000 on the left side during clenching, and a higher activation of digastric with p-value being 0.001 on the right side and 0.003 on the left side during swallowing. The mean value showed a statistically significant difference between cases and controls (Table 4).
|
|
Case/Controls |
N |
Mean Rank |
Sum of Ranks |
P value |
|
Digastric Right-Side Clench |
Cases |
30 |
24.91 |
722.5 |
0.001 |
|
Controls |
12 |
11.54 |
138.5 |
||
|
Dgastric Left Side Clench |
Cases |
30 |
24.02 |
696.5 |
0 |
|
Controls |
12 |
13.71 |
164.5 |
||
|
Dgastric Right Side Swallon |
Cases |
30 |
23.97 |
695 |
0.001 |
|
Controls |
12 |
13.83 |
166 |
||
|
Dgastric Left Side Swallon |
Cases |
30 |
22.17 |
643 |
0.03 |
|
Controls |
12 |
18.17 |
218 |
||
|
Dgastric Right Side Resting |
Cases |
30 |
25.97 |
753 |
0 |
|
Controls |
12 |
9 |
108 |
||
|
Dgastric Left Side Resting |
Cases |
30 |
26.16 |
758.5 |
0 |
|
Controls |
12 |
8.54 |
102.5 |
Table 4: Mann Whitney Test Comparing Masseter EMG at Clench Resting and Swallow between Cases and Controls.
A higher activation of sternocleidomastoid with p-value 0.000 on the right side and 0.000 on the left side during rest. A higher activation of sternocleidomastoid with p-value 0.001 on the right side and 0.003 on the left side during clenching, and a higher activation of sternocleidomastoid with p-value being 0.000 on the right side and 0.05 on the left side during swallowing. The mean value showed a statistically significant difference between cases and controls (Table 5).
|
|
Case/Controls |
N |
Mean Rank |
Sum of Ranks |
P value |
|
Scm Right Side Clench |
Cases |
30 |
23.14 |
671 |
0.001* |
|
Controls |
12 |
15.83 |
190 |
||
|
Scm Left Side Clench |
Cases |
30 |
24.45 |
709 |
0.003* |
|
Controls |
12 |
12.67 |
152 |
||
|
Scm Right Side Swallow |
Cases |
30 |
23.72 |
688 |
0.000* |
|
Controls |
12 |
14.42 |
173 |
||
|
Scm Left Side Swallow |
Cases |
30 |
24.29 |
704.5 |
0.05* |
|
Controls |
12 |
13.04 |
156.5 |
||
|
Scm Right Side Resting |
Cases |
30 |
25.45 |
738 |
0.00* |
|
Controls |
12 |
10.25 |
123 |
||
|
Scm Left Side Resting |
Cases |
30 |
25.22 |
731.5 |
0.00* |
|
Controls |
12 |
10.79 |
129.5 |
Table 5: Mann Whitney Test Comparing Sternocleidomastoid EMG at Clench Resting and Swallow between Cases and Controls.
Balancing of muscle bilaterally in % is evaluated during clenching and swallowing. With a percentage of balancing in temporalis during clenching being 17.55% and 16.38% during swallowing, masseter 18.45 % during clenching and 16.98% during swallowing, digastric 15.09 % during clenching and 15.97% during swallowing and sternocleidomastoid 15.60% during clenching and 16.38% during swallowing (Table 6).
|
|
Cases/ Controls |
N |
Mean Rank |
Sum of Ranks |
P value |
|
Temporalis Balancing % on Clench |
Cases |
30 |
17.55 |
509 |
0.003* |
|
Controls |
12 |
29.33 |
352 |
||
|
Temporalis Balancing % on Swallon |
Cases |
30 |
16.38 |
475 |
0.000* |
|
Controls |
12 |
32.17 |
386 |
||
|
Masseter Balancing % on Clench |
Cases |
30 |
18.45 |
535 |
0.000* |
|
Controls |
12 |
27.17 |
326 |
||
|
Masseter Balancing % on Swallon |
Cases |
30 |
16.98 |
492.5 |
0.001* |
|
Controls |
12 |
30.71 |
368.5 |
||
|
Digastric Balancing % on Cench |
Cases |
30 |
15.09 |
437.5 |
0.00* |
|
Controls |
12 |
35.29 |
423.5 |
||
|
Diagstric Balancing % on Swallon |
Cases |
30 |
15.97 |
463 |
0.00* |
|
Controls |
12 |
33.17 |
398 |
||
|
Scm Balancing % on Clench |
Cases |
30 |
15.6 |
452.5 |
0.00* |
|
Controls |
12 |
34.04 |
408.5 |
||
|
Scm Balancing % on Swallon |
Cases |
30 |
16.38 |
475 |
0.00* |
|
Controls |
12 |
32.17 |
386 |
Table 6: Mann Whitney Test Comparing Balancing % Of Temporalis, Masseter, Digastric And Scm On Clench And Swallow Between Cases And Controls
Discussion
Swallowing is one of the most important oral functions. This action involves numerous head and neck muscles as well as cortical and subcortical systems [31,32]. Swallowing function starts in embryonic life and its kinematic is age related [33]. Swallowing has attracted an enormous amount of attention over the past few decades due to its involvement in numerous bodily functions, including respiration, mastication, and movement [34]. Many authors provided outstanding explanations of its auxiliary role [35]. Multiple research studies reveal that the duration of the oral phase of saliva swallowing muscle activity and the duration of the saliva swallowing time vary between normal and unphysiological swallowing [24,26].
The spontaneous and involuntary acts that cause the activation of the masticatory muscles, the closing of the mouth, and the temporomandibular stabilization make this type of oral function crucial for both swallowing and jaw to cranium stabilization [27].
In patients who are suffering from TMD, the most common oral parafunctions habits that are diagnosed are clenching and grinding [36] such parafunctional habits are commonly associated with TMD patients who are suffering from headaches [3]. Michelotti et al found that oral parafunctions (clenching/grinding) were more frequent among TMD patients than healthy subjects,18 while Anderson GC et al found a correlation between TMD , temple headache and myofascial pain [38]. Few studies have evaluated the correlation between swallowing and temporomandibular disorders [39-40]. Weber et al evaluated the position of the mandible and the hyoid bone in patients with and without TMD. Winocur E et al have concluded that patients with abnormal mastication and deglutition had a higher incidence of TMDs and during swallowing; TMD patients demonstrated an exerted effort in positioning of their lips and tongue [41]. In another study conducted by Fassicollo, C. E et al there was an increased activity of anterior temporalis and masseter muscles during saliva swallowing among patients with TMD, but no difference in activity of submental muscles when compared to healthy individuals was observed [42].
In the present paper, we have evaluated correlation between TMD and swallowing during rest, clenching, and swallowing using 4-channel sEMG with Biopak software. The study group revealed presence of hyperactivity associated with temporalis muscle, masseter muscle, digastric muscle, and sternocleidomastoid muscle bilaterally and the p-value was ≥0.05, which was considered significant. An increased masticatory muscles activity is correlated with modifications of tongue position within the oral cavity [43]. Which may further alternate the masticatory muscles performance [43].
Through our study we would like to emphasis that due to hyperactivity of muscles of head and neck an individual may undergo compensatory motor adjustments for deglutition leading to dysphagia. This leads to increased positive airway pressure in individuals with high electrical activity of muscles of head and neck which may precede to a new category of OSA known as “dysphagic OSA” (Figure 3).
Figure: 3 Association between TMD and dysphagic OSA.
The impact of TMD along with OSA have a significant influence on the quality of life of affected individuals [43]. Both the conditions manifest in distinct ways, leading to a range of physical, emotional, and cognitive challenges [43]. Recognizing the signs, symptoms, and cause of TMD and OSA are the crucial steps towards effective management. Timely intervention and lifestyle modification may mitigate the negative impact of these disorders [43].
The limitations of the present results are related to the design of the study, which did not permit us to compare the electrical activity between surface EMG(records electrical activity of muscles occurring on the skin surface) and intramuscular EMG (records electrical activity occurring withing fibers of the muscles) [44]. Variability sEMG measurements may occur within individuals across session as well as during repetition of the task as the result of slight variation in placement of electrodes which may alter signal conductivity [44].
However, future studies can be more scientifically accurate by including foods of different consistencies, measurement of strength of tongue and evaluating patients with videofluroscopy would permit us to define with precision different phases and duration of swallowing associated to TMD [44].
Conclusion
The oral parafunctional habits have an impact in TMD pathogenesis. The most common parafunctional habits correlated to TMD are clenching and griding. In the present paper, we have evaluated activity facial muscles during rest, clenching, and swallowing using sEMG in patients suffering from TMD and observed hyperactivity of temporalis muscle, masseter muscle, digastric muscle, and sternocleidomastoid muscle bilaterally. Which when associated with long-term undiagnosed OSA may lead to “dysphagic OSA”.
The integration of 4-channel EMG in the assessment of muscles in the head and neck region has advanced our understanding of the neuromuscular aspects of TMD. The findings from this study underscore the importance of early detection, individualized treatment plan multidisciplinary approach, and the management of parafunctional habits to optimize the outcomes and quality of life for TMD patients. Further research in this area will undoubtedly contribute to the refinement of TMD diagnostics and treatment modalities.
References
- Greene CS, Mogini F. (2005) Temporomandibular disorders (TMD). Prog Orthod. 6:224-31.
- Scrivani SJ, Keith DA, Kaban LB. (2008) Temporomandibular disorders. N Engl J Med. 359:2693-705.
- Gesch D, Bernhardt O, Alte D, Schwahn C, Kocher T, et al. (2004) Prevalence of signs and symptoms of temporomandibular disorders in an urban and rural German population: results of a population-based Study of Health in Pomerania. Quintessence Int. 35:143-50.
- List T, Wahlund K, Wenneberg B, Dworkin SF. (1999) TMD in children and adolescents: prevalence of pain, gender differences, and perceived treatment need. J Orofac Pain 13:9-20.
- Gupta B, Thumati P, Radke J. (2016) Temporomandibular joint vibrations from totally asymptomatic subjects. CRANIO®. 34(3):169-75.
- Maixner W, Greenspan JD, Dubner R. (2011) Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case–control study. J Pain 12(suppl): T75-91.
- Manfredini D, Guarda-Nardini L, Winocur E, Piccotti F, Ahlberg J et al. (2011) Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112:453-62.
- Young DM. (1999) Acute pain management protocol. J Gerontol Nurs, 25:10–21.
- Barghan S, Tetradis S, Mallya S. (2012) Application of cone beam computed tomography for assessment of the temporomandibular joints. Aust Dent J. 57(suppl 1):109-18.
- Greene CS, Klasser GD, Epstein JB. (2010) Revision of the American Association of Dental Research’s science information statement about temporomandibular disorders. J Can Dent Assoc. 76: a115.
- Suvinen TI, Kemppainen P. (2007) Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil. 34: 631-44.
- Belfer ML, Kaban LB. (1982) Temporomandibular joint dysfunction with facial pain in children. Pediatrics. 69:564-67.
- Gay T, Bertolami CN, Donoff RB, KEITH D A, Kelly J P, et al. (1987) The acoustical characteristics of the normal and abnormal temporomandibular joint. J Oral Maxillofac Surg. 45:397-407.
- Kaplan A, Buchbinder D, McCormick S. (1991) Temporomandibular disorders, and facial pain. Mt Sinai J Med. 58:229-34.
- Sanders AE, Essick GK, Fillingim R. (2013) Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res. 92(suppl):70S-0S.
- Bettega G, Pepin JL, Levy P. (1998) Surgical treatment of a patient with obstructive sleep apnea syndrome associated with temporomandibular joint destruction by rheumatoid arthritis. Plast Reconstr Surg. 101:1045-50.
- Zardi FT, Nagalaxmi V, Gupta B, Reddy R, Rao SE. (2024) Sleep Disorders & Bruxism–Trigeminal Cardiac Reflex a Missing Link. Saudi J Oral Dent Res. 9(7):122-8.
- Michelotti A, Cioffi I, Festa P, Farella M. (2010) Oral parafunctions as risk factors for diagnostic TMD subgroups. J Oral Rehabil. 37:157-62.
- Jensen R, Olesen J. (1996) Initiating mechanisms of experimentally induced tension-type headache. Cephalalgia. 16:175-82.
- Wieckiewicz M, Grychowska N, Wojciechowski K, Pelc A, Zietek M, et al. (2014) Prevalence and correlation between TMD based on RDC/TMD diagnoses, oral parafunctions and psychoemotional stress in Polish university students. Biomed Res Int. 472346.
- Mc CD. (1949) Open bite and tongue-thrusting. Am J Orthod. 35:377-80.
- Speidel TM, Isaacson RJ, Worms FW. (1972) Tongue-thrust therapy and anterior dental open-bite. A review of new facial growth data. Am J Orthod. 62:287-95.
- Kalia AJ. (2017) Treatment of anterior open bite with a mini-implant-supported tongue crib. J Clin Orthod. 51:37-45
- Vaiman M, Eviatar E, Segal S. (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 1. Quantitative data: timing measures. Otolaryngol Head Neck Surg. 131:548-5.
- Vaiman M, Eviatar E, Segal S. (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 2. Quantitative data: amplitude measures. Otolaryngol Head Neck Surg. 131:773-80.
- Vaiman M, Eviatar E, Segal S. (2004) Surface electromyographic studies of swallowing in normal subjects: a review of 440 adults. Report 3. Qualitative data. Otolaryngol Head Neck Surg. 131:977-85.
- Ciavarella D, Mastrovincenzo M, Sabatucci A. (2010) Effect of the Enveloppe Linguale Nocturne on atypical swallowing: surface electromyography and computerised postural test evaluation. Eur J Paediatr Dent 11:141-45.
- Ingervall B. (1978) Activity of temporal and lip muscles during swallowing and chewing. J Oral Rehabil. 5:329-37.
- Stegeman DF, Blok JH, Hermens HJ, Roeleveld K. (2000) Surface EMG models: properties and applications. Journal of Electromyography and Kinesiology.10(5):313-26.
- Okeson J. (1996) Oro-Facial Pain: Guideless for Assessment, Diagnosis, and Management. Chicago, IL: Quintessence Publishing Co.
- Buchholz DW, Bosma JF, Donner MW. (1985) Adaptation, compensation, and decompensation of the pharyngeal swallow. Gastrointest Radiol. 10:235-39.
- Ertekin C. (2011) Voluntary versus spontaneous swallowing in man. Dysphagia. 26:183-92.
- Humbert IA, Fitzgerald ME, McLaren DG, Johnso S, Kosmatka EPK, et al. (2009) Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 44:982-91.
- Schindler JS, Kelly JH. (2002) Swallowing disorders in the elderly. Laryngoscope. 112:589-602.
- Vaiman M, Shoval G, Gavriel H. (2008) The electrodiagnostic examination of psychogenic swallowing disorders. Eur Arch Otorhinolaryngol. 265:663-8.
- Winocur E, Gavish A, Finkelshtein T, M Halachmi, E Gazit, et al. (2001) Oral habits among adolescent girls and their association with symptoms of temporomandibular disorders. J Oral Rehabil. 28:624-29.
- Jensen R, Rasmussen BK. (1996) Muscular disorders in tension-type headache. Cephalalgia. 16:97–103.
- Anderson GC, John MT, Ohrbach R, Nixdorf DR, Schiffman EL, et al. Influence of headache frequency on clinical signs and symptoms of TMD in subjects with temple headache and TMD pain. Pain. 152(4):765-71.
- De Felicio CM, De Oliveira MM, Da Silva MA. (2010) Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio. 28:249-59.
- Oltramari-Navarro PV, Yoshie MT, Silva RA, Fernandes KBP, Navarro R de L, et al. (2017) Influence of the presence of temporomandibular disorders on postural balance in the elderly. Codas. 29:e20160070.
- 41. Winocur E, Gavish A, Finkelshtein T, et al. (2001) Oral habits among adolescent girls and their association with symptoms of temporomandibular disorders. J Oral Rehabil. 28:624-9.
- Fassicollo CE, Machado BCZ, Garcia DM, de Felício CM. (2018). Swallowing changes related to chronic temporomandibular disorders. Clinical Oral Investigations. 3287-96.
- Kollias I, Krogstad O. (1999) Adult craniocervical and pharyngeal changes—a longitudinal cephalometric study between 22 and 42 years of age. Part II: Morphological uvulo-glossopharyngeal changes. Eur J Orthod. 21:345-55.
- Steele C. (2015) The blind scientists and the elephant of swallowing: a review of instrumental perspectives on swallowing physiology. J Texture Stud.

