Antifungal Susceptibility Pattern of Seed Exracts of Ricinodendron Heudelotii (njangsa) on Candida Albicans and Aspergillus Niger
Esoh Rene Tanwieh1, Solomon Gyampoh2 and TIDOH KENNE Linda Nicaise3
1University affiliation: University of Bamenda-Cameroon Faculty : FHS (faculty of health sciences), Department : Medical and biomedical sciences, Specialty: Medical laobarotary sciences, Cameroon
2School of Pharmaceutical Sciences, Phagwara, Lovely Professional University, India
3Medical laobarotary Department At Training school for senior laboratory technicians Cameroon
*Corresponding author: Esoh Rene Tanwieh, University affiliation: University of Bamenda-Cameroon Faculty : FHS (faculty of health sciences), Department : Medical and biomedical sciences, Specialty: Medical laobarotary sciences, Cameroon
Citation: Tanwieh ER, Gyampoh S and Linda Nicaise TK. (2024) Antifungal susceptibility pattern of seed exracts of Ricinodendron heudelotii (njangsa) on Candida albicans and Aspergillus niger. Adv Clin Med Res. 5(3):1-21.
Received: March 1, 2024 | Published: March 21, 2024
Copyright© 2024 genesis pub by Tanwieh ER, et al. bCC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Abstract
The search for new substances with antifungal activities has become an urgent necessity due to resistance against antifungal by many fungi of medical importance. In attempt to sought out new antifungal agents from plants, susceptibility of Candida albicans and Aspergillus niger to the seed extracts of Ricinodendron heudelotii was assessed. Qualitative phytochemical screening was also done to establish the various phytochemicals found in the plant. To achieve these, the dried seeds of Ricinodendron heudelotii were collected during the dry season in Bamenda food market, and ground. The powder obtained was subjected to aqueous and alcoholic extractions separately.The alcoholic and aqueous extracts of Ricinodendron heudelotii seeds were evaluated for their antifungal potential against C albicans and A neger using agar dilution method. Results obtained indicated that, C albicans was susceptible to the alcoholic extract while both isolates were resistant to the aqueous extract.
Phytochemical screening results indicated the presence of resins and saponins, while alkaloids, glycosides, tannins and flavonoids are absent. In conclusion, the alcoholic extract of R heudelotii seeds has antifungal activity only against C albicans and the aqueous extraction does not show any antifungal activity against the chosen isolates. It was proposed that further studies should be done on the safety of the extracts in-vivo.
Keywords
Aspergillus niger; Aqueous extract; Deoxyribonucleic acid; Traditional Medicine
List Of Abbreviations
A. neger: Aspergillus niger; AIFCR: American Institute for Cancer Research; Alc. Ext: Alcoholic Extract; Aq. Ext: Aqueous Extract; C. albicans: Candida albicans; DNA: Deoxyribonucleic Acid; HIV: Human Immunodeficiency Virus SDA: Sabaroud Dextrose Agar; UNESCO: United Nations Educational, Scientific and Cultural Organization; WCRF: World Cancer Research Fund; WHO: World Health Orgnization.
Introduction
In recent years, the use of plants in traditional medicine has increased the interest in ethno botanical studies throughout the world. In fact, the World Health Organization (WHO) estimated that 80 % of the population of the developing countries relies on traditional medicine using medicinal plant for their primary health care need [1].
In Cameroon, medicine is still unorganized, making it integration in the health system ineffective [2].The interest in herbal medicine in Cameroon has progressed similarly to increased interest in other developing countries. Recently, various studies [3-5] have been conducted to prevent the folk medicine from disappearing. For centuries, people have been using herbal medicine for the treatment of some common ailments [6].
Plants have been reported to contain many biologically active compounds, which have potential development as medicinal agents. Phytochemical progress has been aided by the development of rapid and accurate methods of screening plants for particular chemicals (Banso, 2009). The screening of higher plants for purposes represents a serious effort to discover newer, safer, and possibly more effective drugs with potential of fighting pathogenic microbes [7].
There is no doubt that we are blessed and surrounded by countless plants and trees that offer countless benefits to us [8]. However, the call to action is for all of us to regularly acknowledge these amazing plants so as to completely gain all the benefits they offer to us. One of such plants that are of great importance but yet under-utilized is the Ricinodendron [8]. Ricinodendron heudelotii, the only species of the genus Ricinodendron belong to the kingdom Plantae, order Malphighiales, family Euphorbiaceae and two varieties of tree sub- species are recognized: the sub-species heudelotii which is found from Senegal to Benin and the sub-species africanum found in South and East of Nigeria [8].
Ricinodendron heudelotii is a fast-growing tree that is originally from Africa, precisely Liberia, Tanzania, Senegal, Angola, Mozambique, Sudan and Cameroon [8]. The edible parts of the plant are the high nutritive contents of the njangsa seeds. The dried and ground seeds are used as a flavoring agent in some dishes in west and central Africa. The paste of the ground seed is used to thicken soups and stew. The oil extracted from the njangsa seeds has a yellowish color and tastes similar to groundnut oil. Because of its high content of y- tocopherol¸ the oil is very stable and it becomes rancid only slowly. This oil is interesting as cooking oil and margarine [9]. Apart from being used as local spice, the njangsa oily seed is also used for several medicinal purposes. Thus, the aim of this study was to determine the antifungal activity of it seed extracts on Candida albicans and Aspergillus niger.
The edible parts of the plant are the high nutritive contents of the njangsa seeds. The dried and ground seeds are used as a flavoring agent in some dishes in west and central Africa. The paste of the ground seed is used to thicken soups and stew. The oil extracted from the njangsa seeds has a yellowish color and tastes similar to groundnut oil. Because of its high content of y- tocopherol¸ the oil is very stable and it becomes rancid only slowly. This oil is interesting as cooking oil and margarine [9]. Apart from being used as local spice, the njangsa oily seed is also used for several medicinal purposes. Thus, the aim of this study was to determine the antifungal activity of it seed extracts on Candida albicans and Aspergillus niger.
Statement of the problems
Resistances against antifungal by many fungi are accumulating. Therefore, the search for new substances with antifungal activity has become an urgent necessity [10]. Medicinal plants are frequently used in popular medicine as remedies for many infectious diseases. In many countries of Africa and other places in Cameroon, a good number of persons have been found consuming the seeds of R heudelotii. Due to this observation, the researchers were interested on investigating whether the seeds plant has antimicrobial properties against some microbes of medical importance.It is therefore important to know the effects of seed extracts of Ricinodendron heudelotii (njangsa) on Candida albicans and Aspergillus niger.
Research question
What is the effect of the seed extracts of Ricinodendron heudelotii (njangsa) on Candida albicans and Aspergillus niger?
Research objectives
General objective
To determine the effect of seed extracts of Ricinodendron heudelotii on Candida Albicans and Aspergillus niger.
Specific objectives
To establish the susceptibility of some pathogenic fungi to Ricinodendron heudelotii seed extracts. To carry out agar dilution technique using Ricinodendronheudelotii seed extracts. To determine the different phytochemicals, present in Ricinodendron heudelotii seeds.
Hypothesis
Seed extracts of Ricin dendron heudelotii have antimycobial effects on Candida albicans and Aspergillus niger.
Impact of the study
Our outcome of this study is to enable us to better understand the important biological components found in Ricin dendron heudeloti and promote knowledge concerning the use of medicinal plants in the world and in Cameroon in particular. This may also enable us to use the seeds as a less costly product for the treatment of candidiasis.
Limitation
The study is carried out on two pathogenic fungi Candida albicans, Aspergillus niger and no bacteria involved.
Literature Review
Antimicrobial activity of medicinal plants
Medicinal plants are sources of important quantities of chemical substances which are able to initiate different biological activities including those useful in the treatment of human diseases (Etoaet al., 2016). Even though pharmacological industries have produced a number of new antifungals in the last three decades, resistance to these drugs by fungi has increased.
The number of multi-drug resistant microbial strains and the appearance of strains with reduced susceptibility to antibiotics are continuously increasing. This increase has been attributed to indiscriminate use of broad-spectrum antibiotics, immunosuppressive agent, intravenous catheters, organ transplantation and ongoing epidemics of HIV infection [11].
In addition, in developing countries, synthetic drugs are not only expensive and inadequate for the treatment of diseases but also often with adulterations and side effects. Despite the existence of potent antibiotics, resistant or multi-resistant strains are continuously appearing, imposing the need for a permanent search and development of new drugs. For Centuries plants have been used throughout the world as drugs and remedies for various diseases [12]. Plants are considered not only as dietary supplement to living organisms but also traditionally used for treating many health problems and the medicinal value of many plants still remains unexplored. Investigations of plants are carried out to find novel drugs or templates for the development of new therapeutic agents [13]. Over 60% of the world human population, 80% in developing countries depends directly on plants for their medicinal purposes [14]. The use of plant’s compounds for pharmaceutical purposes has gradually increased. The inclusion of traditionally used medicines including phytomedicine if they prove safe and effective into national health care system is suggested by World Health Organization [1]. Therefore, plants should be investigated to better understand their properties, safety and efficacy.
Traditional medicine in Africa
African traditional medicine is the oldest and perhaps the most diverse system. Africa is considered to be the cradle of mankind, with a rich biological and cultural diversity and there are many differences between different regions of this continent when it comes to healing practices [16]. The tradition of collecting plants as well as processing herbal remedies and applying them has been handed down from generation to generation. Traditional medicine is an important part of the health care system in most of the African countries. About 80-90% of the populations of African countries are dependent on traditional medicine for their primary health care [16]. For example, in Soudan, traditional medicine plays an important role for health care, since hospitals and other medicinal facilities are limited and high percentage of the population are nomads [17].
Traditional medicine seems to have certain advances over modern system of medicine because it is an integral part of the people’s culture and is particularly effective in solving certain cultural health problem [18]. In Cameroon, although [19] provided a useful review of the traditional use of medicinal plants, much work remains to be done regarding the documentation of existing ethnobotanical knowledge [6] also documented the traditional use of 289 plants species belonging to 89 families against 220 pathologies. 68% of the documented plants are used to treat more than twenty important diseases. They are used as decoction, infusion, maceration, powder, powder mixtures, plaster, calcinations, and squeeze in water, boiling, cooking with young cock or sheep meat or groundnut paste, direct eating, juice, fumigation, and sits bath [6]. The most recurrent diseases or disorders treated are typhoid, male sexual disorders, malaria, gonorrhea, gastritis, rheumatism, fever, dysentery, diarrhea, dermatitis, boils, cough, wounds, syphilis, sterility, sexually transmitted diseases, ovarian cysts, and amoebiasis, with more than two hundred plants being used to cure these diseases or disorders [6].
Some fungi of medical importance
|
Name of fungus |
Source |
Involvement |
Disease and initiation |
|
Candida albicans |
Ubiquitous and occur naturally on humans |
Mouth, throat, skin, vagina, fingers, nails, bronchi, lungs, gastrointestinal tract, occasionally systemic. |
Candidiasis: due to impaired epithelial barrier functions. |
|
Tinea species |
Soil, animals, man. |
Skin, hair, nails, fingers, feet, shaft. No living tissue is invaded. |
Dermatophytosis: traumatic implantation of the aetiologic agent. |
|
Cryptococcus neoformans |
Can be isolated from dung of caged birds (canaries, parrots) and |
Pulmonary, systemic or meninges. |
Cryptococcis: initiated by the inhalation of basidiospores and/or desiccated |
|
Aspergillus |
Cosmopolitan, |
Skin, nails, body |
Aspergillosis: |
|
Histoplasma |
Soil enriched |
Pulmonary, chronic |
Histoplasmosis: |
Table1: Some fungi of medical importance.
Diseases caused by Candida albicans and Aspergillus niger
Candidiasis
Candida albicans is the commonest cause of candidiasis (moliniasis). The yeast is a common commensal of gastrointestinal tract. Most Candida infections are opportunistic, occurring in debilitated persons. Candidiasis is also associated with prolong broad spectrum antibiotic therapy [20]. Many different clinical forms of candidiasis are known, involving primarily the mucosal surfaces (thrush), gastrointestinal or urogenital tracts and deep-seated infections such as candideamia or meningitis. Candida vaginitis is a common infection during pregnancy [20]. Candida infections of the mouth and oesophagus occur in 80-95% of those with HIV disease [20].
Aspergillosis
Aspergillus niger is a saprophyte mould. Most live in the environment without causing disease. Disease caused by aspergillus include allergic bronchopulmonary aspergillosis (causes asthma and eosinophilia) due to hypersensitivity to aspergillus antigen inhaled in airbone conidia; aspergilloma in which inhaled conidia germinate in a pulmonary cavity and grow into a “fungus ball” and; invasive aspergillosis in which the fungus infects the lung (causing acute pneumonia) and spread to other organs, producing abscesses and necrotic lesions. Invasive aspergillosis occurs mainly in immunocompromised patients and is often life threatening [20].
Ricinodendron heudelotii
Geographical distribution of Rrcinodendronheudelotii
Ricinodendron heudelotii is common in tropical regions. The native geographic location of “njangsa” reaches from Senegal in West Africa to Sudan, Uganda and Tanzania and from Sudan down to the western coast of Sub-Sahara Africa to Angola. The tree is also found in Madagascar and Cameroon where the main production area is the humid forest zone [21].
Ricinodendron heudelotii grows generally in rain forests and is also typical for secondary forests. This tree is a light- demanding species [21]. Therefore, it can also be found in deciduous forests, forest edges, secondary scrubs and in semi-dry savannahs. The tree is observed in food crop fields, cocoa farms and other agro-forestry systems, where the tree can also intentionally be planted [9].
Taxonomy
Kingdom: Plantae; Phylum: Tracheophyta; Class: Magnoliopsida; Order: Malpighiales; Family: Euphorbiaceae; Sub-Family: Crotonoideae; Tribes: Ricinodendreae; Genus: Ricinodendron; Species: R. heudelotiis; Binomial name: Ricinodendron heudelotii; Subspecies: R heudelotii heudelotii R heudelotii africanum; [22].
Synonyms
Jatropha heudelotii baill [22].
Common names
French: boisjasanga, essang, essessang.
English: African nut-tree, African oil-nut-tree, African wood oil-tree, ezang, njangsa/ njangsang, Ricinodendron [22].
Vernacular names
In Angola, it is called Mugella; In Cameroon, it is called Essessang; In Zaire it is called Bofeko; In Ghana, it is called Wama; In Nigeria, it is called Okhuen; In Uganda, it is called Kihongo; In Coted’ivoire, it is called Akpi [21].
Description of Ricinodendron heudelotii
Ricinodendron heudelotii is a dioecic plant found in central and West Africa. The tree is fast growing and reaches a height between 20 and 50m with straight trunk which can have a diameter up to 2.7m. His crown is broad and the roots are widely spread. The bark is smooth with a grey color. Inside, the bark is red when cut [21].
The flowers are yellowish-white, 5mm long and form a long terminal panicle which measures between 15 and 40cm. Flowering time is between April and May. Male panicles are longer and slender than female flowers [21].
Njangsa trees produce fruits that are typically 2-3 lobed and contain 2 cells in which the seeds lie. These seeds are red-brown to black in color, rounded and some 1cm in diameter. The seeds are oily in texture and can be bought either raw or dried. They have an odor reminiscent of oily chocolate, but their flavor is truly unique: subtly aromatic with a mild bitter taste [21]. At maturity (August- September) the fruits smell like over-ripe apples [21].
How to Extract the njangsa Seeds?
Extracting the njangsa seeds from the fruits can be quite demanding. The big green kidney-shaped fruits are normally gathered into piles under the tree. The fruits are left for up to four weeks to decompose so that the fleshy parts can easily peel off [23].
The nuts are then extracted from the rotten fruits after which they are boiled for a long time. Once the nuts are a bit softened, they can then be cracked for the inner kernels to be dried. The dried njangsa kernels can be kept for years and used as desired [23].
Chemical Constituents of Ricinodendron heudelotii seeds
The medicinal plants are useful for healing as well as for curing human diseases because of the presence of phytochemical constituents. Phytochemicals are non- nutritive chemicals that have protective or disease preventive properties [24]. They are non-essentials nutrients present in plants leaves, roots, fruits, vegetables and back of trees. A compositional analysis of “njangsa” revealed a presence of long chain omega-3 fatty acids not usually associated with plant material. The seed has 31.4% crude protein and 44.7% lipid [25]. Of this lipid, about73% is composed of polyunsaturated fatty acids, almost entirely of eicosapenteanoic acid, with about 18% oleic acid [25].
The seed also contains about 8.9% of crude fibers, 5.6% of carbohydrates with 495kcal being the energetic value per 100g of seeds [9]. The phytochemical analysis of the oil extracts from the seeds showed the presence of the following minerals: Na, K, Ca, Mg, Mn, Fe, Cu and Zn as well as the following family of compounds: steroids, saponins, and terpenoids (Mordi set al, 2016). Phytochemicals play many rules in the body.
Functions of phytochemicals in the body
Researchers have found that phytochemicals have the potential to stimulate the immune system, prevent toxic substances in the diet from becoming carcinogenic, reduce inflammation, prevent DNA damage and aid DNA repair, reduce oxidative damage to cells, slow the growth rate of cancer cells, trigger damage cells to self- destruction (apoptosis) before they can reproduce, help regulate intracellular signaling of hormones and gene expressions, and activate insulin receptor [26,27]. Some major functions are:
- Antioxidant: Most phytochemicals have antioxidant activity and protect our cells against oxidative damage and reduce the risk of developing certain types of cancer. Phytochemicals with antioxidant activity: allyl sulfides (onions, leeks, garlic), carotenoids (fruits, carrots), flavonoids (fruits, vegetables), polyphenols (tea, grapes) (Biesalki, 2008).
- Hormonal action: Isoflavones, found in soy, imitate human estrogens and help to reduce menopausal symptoms and osteoporosis (Biesalki, 2008).
- Stimulation of enzymes: Indoles, which are found in cabbages, stimulate enzymes that make the estrogen less effective and could reduce the risk of breast cancer. Other phytochemicals, which interfere with enzymes, are protease inhibitors (soy and beans), terpenes (citrus fruits and cherries) (Biesalki, 2008).
- Interference with DNA replication: Saponins found in beans interfere with the replication of cell DNA, thereby preventing the multiplication of cancer cells. Capsaicin, found in hot peppers, protects DNA from carcinogens (Biesalki, 2008).
- Anti-bacterial effect: The phytochemical allicin from garlic has anti-bacterial properties (Biesalki, 2008).
- Physical action: Some phytochemicals bind physically to cell walls thereby preventing the adhesion of pathogens to human cell walls. Proanthocyanidins are responsible for the anti-adhesion properties of cranberry. Consumption of cranberries will reduce the risk of urinary tract infections and will improve dental health (Biesalki, 2008).
Brief study of some secondary metabolites
Alkaloids
Alkaloids are complex compounds, basic in nature, defined by the amine function, which provides its constituent chemical properties that are related to a high toxicity. Alkaloids belonging to beta-carboline group possess antimicrobial, anti- HIV and anti-parasitic activities [28]. They also act as proteins synthesis, protective substances against animals and insects, detoxicating agents of the plant [29].
Flavonoids
Flavonoids are the pigments that color most flowers, fruits, and seeds. They are secondary metabolites, widely distributed in plants [30]. Flavonoids are powerful antioxidants with anti-inflammatory and immune system benefits.
Glycosides
Glycosides are basically molecules where in a sugar is attached to a non- carbohydrate element, generally a minor natural molecule. They are soluble in water and alcohol and have several important functions in all living organisms. A number of plants stock up compounds in the form of dormant glycosides. These chemicals may be set in motion by certain enzymes by means of hydrolysis that results in the sugar portion to be detached. The activation of these chemicals by the enzymes makes them accessible for utilization. A number of such glycosides enclosed by the plants are of therapeutic value [31].
Tannins
Tannins are water-soluble polyphenols that are commonly found in higher herbaceous and woody plants [32] mainly in leaf, bud, seed, root, and stem tissues. They can be classified into two categories: hydrolysable and non- hydrolysable (condensed). Tannic acid is an important gallotannin belonging to the hydrolysable class, while catechin belongs to the non-hydrolysable class. Hydrolysable tannins are esters of phenolic acids and a polyol, usually glucose [33]. It has been reported that tannic acid and propyl gallate, but not gallic acid, were inhibitory to foodborne bacteria, aquatic bacteria, and off- flavor-producing microorganisms. Their antimicrobial properties seemed to be associated with the hydrolysis of ester linkage between gallic acid and polyols hydrolyzed after ripening of many edible fruits. Tannins in these fruits thus serve as a natural defense mechanism against microbial infections. They inhibit the growth of many fungi, yeasts, bacteria, and viruses [33]. They also play a role in protection from predation, and perhaps also as pesticides, and in plant growth regulation [34].
Resins
Resins are "solid or highly viscous substances," which are typically convertible into polymers. Such viscous substances can be plant-derived or synthetic in origin. They are often mixtures of compounds. The medical action of resins vary enormously. Generally speaking, most resins are antimicrobial and wound healing in animals and in the plants that secrete them. Topical application of pine resins to wounds and burns has been shown to stimulate local immune function, normalized wound hemodynamics, and stimulate squamous epithelization. Beyond this, resins from different plants can have a wide array of effects. for example, frankincense, the gum resin of Boswellia serrata, is well documented as an inflammation modulator that is helpful in asthma and ulcerative colitis; this is attributed at least in part to its inhibition of 5-lipoxygenase. The gum resin of commiphora molmol (myrrh) is antiparasitic, analgesic, and antineoplastic.
Saponin
Saponins are glucosides with foaming characteristics which consist of polycyclic aglycones attached to one or more sugar side chains. The aglycone part, also called sapogenin, is either steroid (C27) or a triterpene (C30) [35]. The foaming ability of saponins is caused by the combination of a hydrophobic (fat-soluble) sapogenin and a hydrophilic (water-soluble) sugar part. Saponins have a bitter taste and are phytochemicals which can be found in most vegetables, beans and herbs. The best-known sources of saponins are peas, soybeans, and some herbs with names indicating foaming properties. Saponins have many health benefits. Studies have illustrated the beneficial effects on blood cholesterol levels, cancer, bone health and stimulation of the immune system.
Traditional uses of the tree
Roots, leaves, back and seeds of the tree have many medicinal uses and they are used in the treatment of ailments with the trunk (back) being the most effective and frequently used part. Many of the medicinal uses range from use as laxative, treatmentof diarrhea, cough, dysentery, aneamia, blennorrhoea, sexual and infertility problem [36].
Roots
The roots or root- back of the “njangsa” tree can be mixed with salt and bush pepper, which is used as a laxative. Laxative helps to stimulate the evacuation of fecal wastes from the bowels. Also, the roots can be mixed together with the back and the decoction used for the treatment of venereal diseases [8].
Back
The back is used by the pregnant women to relieve labour pain and the decoction can be taken by the new mothers to relieve stomachache after birth. It is also used by infusion to prevent miscarriage. The back decoction is used for the treatment of sexual problems, menstrual pains, blennorrhoea and infertility problems [8]. It is used for washing, treating and healing of wounds/sores as well as treatment of rheumatism, aneamia. The bark extract can serve as an antidote for neutralizing poisons: this is because the extracts contain lupeol. The back is also used for the treatment of coughs, catarrh, cold, diarrheoa and dysentery. “njangsa” bark can be ground, warmed and then used for treating elephantiasis [36].
Leaves
Leaves are used for the destruction of worms e.g: extracting guinea- worm. It is also used with the latex as a strong purgative [8].
Seeds
The seeds combined with palm oil to form lotion, can be rubbed on body’s soft spots. “njangsa” seeds can be used for preparing soups to stimulate appetite [36].
Other uses
“Njangsa” tree can be used as a natural manure to improve the soil because the roots are populated by mycorrhizae [9]. The bark, leaves, stems, woods and fruits of the “njangsa” tree can serve as a natural fertilizer. It can be cut and used as stakes for supporting other plants. Fallen njangsa fruits are attractive to animals thus hunters use the tree to guide animals to their game [8].
For constructive purposes, the light white wood of Ricinodendron heudelotii can be used for building and construction. The wood can be used for making toys, cutleries etc. the wood can also be used for electrical insulation. The wood can be carved into plates, spoons, bowls, ladles, pestles, mortars, platters and stools. It is also used for firewood purposes [8].
“Njangsa” seeds can be used as xylophones for generating musical sounds It is also used by villagers for playing local games [9]. The husks and seeds can be processed into oil. Oil processed from dried kernel and wood ash from the burnt “njangsa” tree is suitable for making soap, varnish and indigo dye [9]. The wood ash of the “njangsa” tree can be used as a cooking salt [8].
Materials
The materials used are:
- Fruit material: “njangsa” seeds.
- Instruments: Weighing boat, glass funnel, cotton wool, sieve, grinding machine, sieve, test tubes, Petri dishes, wire loop, measuring cylinder, micropipette, bijou bottles, universal bottles, syringes.
- Chemicals: 95% alcohol (ethanol), distilled water (500ml), Gram stain reagents, 1% aqueous HCl, 6% ferric chloride, iodine solution, concentrated HCl, ammonia solution, Benedict qualitative reagent, dilute NaOH, concentrated H2SO4.
- Test organisms: Candida albicans, Aspergilus. niger.,
- Culture media: Sabaroud Dextrose Agar.
- Equipment: Incubator, balance, microscope, autoclave, fridge, Bunsen burner.
Method
Description of the study area
This research was done in the Science for Life Foundation Laboratory created in 2006 as not-profit organization and located N05°57.921’, E010°09.370’ Nitob IV quarter’s Bamenda II. The mission is to faster community wellness, good environmental stewardship, alleviation of poverty and the building of sustainable live hoods among rural and urban poor. The laboratory bears number of units that is the bacteriology/parasitology, serology, biochemistry, hematology and physiotherapy.
Study design
An experimental research design was used to study the effects of njangsa extracts on C albicans and Aspergillus niger.
Study period
This research was carried out from the 18 january to the 27 Febuary 2017.
Sample preparation and extraction
Well dried njangsa seeds were collected during the dry season in food market Bamenda, it was then grind into powder form by means of a clean and dried machine.
Aqueous extraction
75g of the powder seeds was put in a separate clean and sterile 1000ml conical flask containing 250ml of distilled water. The flask was shaken intermittently for 24 hours. The material was then filtered using Watmann No1 filter paper and the filtrate was transferred into a clean, sterile evaporating dish and was evaporated to dryness in an oven at 500C.
Alcoholic extraction
75g of the powder seeds was put in a separate clean and sterile 1000ml conical flask containing 250 ml of ethanol. The flask was shaken intermittently for 24 hours. The material was then filtered using Watmann No1 filter paper and the filtrate was transferred into a clean, sterile evaporating dish and was evaporated to dryness in an oven at 500C.
Fractionation of the extract
2g of each extract was dissolved into 10ml of distilled water. This gives a concentration of 200mg/ml
Test organisms
Pure cultures of the test organisms were obtained from the Regional Hospital Bamenda (C albicans) and Science for Life Foundation (A niger) where they were identified on the bases of microscopical staining reaction and biochemical tests.
|
Test organisms |
Gram reaction |
Biochemical test |
|
Candida albicans |
Gram positive yeast and pseudohyphae |
Germ tube test |
Table 2: Identification of the test organisms Candida albbicans.
Aspergillus niger
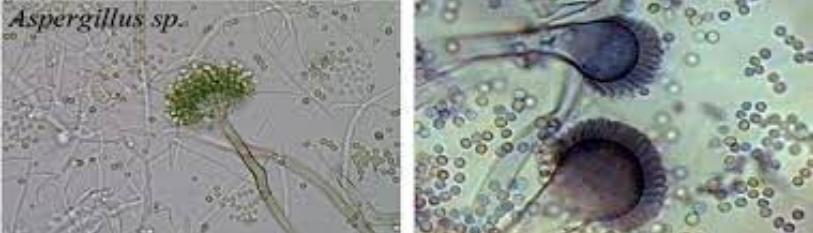
Identification is based on microscopic morphological features stain with Lactophenol cotton Blue (face ventiller) and colonial morphology (black colony on SDA).
Antifungal assay
Inoculum standardization
Small pieces of each pure culture were taken with the help of a wire loop and diluted into 1ml sterile distilled water separately. The test organisms were then adjusted to a turbidity of 0.5 McFarland by adding the pure culture progressively in 1 ml sterile water until it matches with the prepared 0.5 Mcfarland sandard.
Fungal susceptibility tests
The media used (SDA) was prepared according to manufacturer’s instructions after which agar dilution method was performed: the dissolved aqueous extract was well mixed with warmed SDA and the dissolved alcoholic extract was well mixed with heated SDA for the oil to completely dissolve. After this, they were aseptically poured into Petri dishes and allowed to solidify, then dried in the incubator at 400C.
1ml of pure culture of the test micro-organism adjusted to a turbidity of 0.5 McFarland’s standard was used in flooding over Sabaroud Dextrose Agar plates in the agar dilution method of the in-vitro antimicrobial sensitivity test. Six plates were used:
Two plates containing: alcoholic extract+ SDA+ test organisms (C albicans and A niger respectively).
Two plates containing: aqueous extract+ SDA+ test organisms (C albicans and A niger respectively).
Two plates (control plates) containing: SDA+ test organisms (C albicans and A niger).
The plates of Candida albicans were left on bench for one hour and incubated after at 370C while those having Aspergillus niger were incubated at room temperature. All the six plates were incubated for 48 hours and later examined for growth.
Phytochemical analysis
The seed powder of Ricinodendron heudelotii (njangsa) was used as sample for qualitative phytochemical screening for tannins, resins, alkaloids, saponins, glycosides and flavonoids following the standard procedures described by Trease and Evans (1989) and Fon et al., (2009).
Detection of tannins
0.5g of the seed powder was stirred with 10 ml of boiling distilled water and filtered. 0.5ml of 6% ferric chloride was then added to the filtrate. This showed the absence of deep green coloration which indicated the absence of tannins. The second portion of the filtrate was treated with 0.5 ml of iodine solution. This showed the absence of faint bluish coloration which confirmed the absence of tannins.
Detection of resins
5 ml boiling ethanol was added to 0.5 g of the seed powder, the solution was filtered through wathman number 1 filter paper and filtrate was diluted with 4ml of 1% aqueous HCl. The presence of the formation of heaving resinous precipitate indicated the presence of resins.
Detection of alkaloids
0.5 g of the seed powder was stirred with 5ml of 2N HCl in steam water bath, 0.5 ml of wagner’s reagent was then added to the solution previously obtained. This showed the absence of the formation of a precipitate which indicated the absence of alkaloids.
Detection of saponins
0.5 g of fruit powder was stirred with water in a test tube. Frothing was observed on warming which indicated the presence of saponins.
Detection of glycosides
0.5 g of the seed powder was stirred with 10 ml of boiling distilled water then filtered and 2ml of the filtrate obtained was hydrolysed with few drops of ammonia solution. 5 drops of this solution were added to 2 ml of benedict’s qualitative reagent and boiled. The initial (before boiling) blue precipitate remained and this indicated the absense of glycoside.
Detection of flavonoids
0.5 g of the seed powder was dessolved into 2 ml of NaOH solution and few drops of concentrated H2SO4were added. The absence of flavonoids was indicated when the solution remained colored.
Ethical Consideration
An authorization was obtained from the Florence Nightingale Institute of Health and Biomedical Sciences. This document together with the proposal was submitted to the Regional Delegation of Public Health of North West Region for approval. The approved document was given back to Florence Nightingale Institute of Health and Biomedical Sciences and then submitted to the propritor of Science for Life Foundation Laboratory by the researcher for the research to be carried out.
Dissemination of results
After the presentation and corrections of these research findings, the results shall be submitted to the Florence Nightingale Institute of Health and Biomedical Sciences. This will serve as a reference document to the institution and also the ministry of public health to encourage the use of this plant for its medicinal properties.
Results
|
Isolates tested |
Aqueous extract |
Alcoholic extract |
SDA |
|
Candida albicans |
+ |
- |
+ |
|
Aspergillus niger |
+ |
+ |
+ |
Table 3: Susceptibility of test organisms to Ricinodendron heudelotii seed extracts using agar dilution method. {+ = growth (resistance); - = no growth (sensitive)}
Figure 1: Cultures from agar dilution method showing resistance to the aqueous extract of Ricinodendron heudelotii.
Figure 2. Positive control of C albicans and A niger showing growth.
Figure 3: Cultures from agar dilution method showing resistance of A niger and susceptibility of C albicans to ethanolic extract of Ricinodendron heudelotii.
Figure 4: Structures of Ricinodendron heudelotii seeds and fruits (Blessing, 2016).
Figure 5: Picture of Gram-stained smear of Candida albicans (monica, 2006).
Figure 6: Picture of Lactophenol cotton Blue stained of Aspergillus niger (Justine).
The table (Table 3) and figures (Figure1, and 3) above show the results of the susceptibility test of A niger and C albicans to aqueous and alcoholic extracts of Ricinodendron heudelotii seeds using agar dilution method. These results show that the chosen organisms were resistant to the aqueous extract and only C albicans was susceptible to the alcoholic extract of the seeds when comparing to controls (Figure 2).
|
Phytochemical |
Abundance |
|
Tannins |
- |
|
Resins |
++ |
|
Alkaloids |
- |
|
Saponins |
++ |
|
Glycosides |
- |
|
Flavonoids |
- |
|
- = absent + = present but trace ++ = present but less abundant +++ = present abundant |
|
Table 4: Types of compounds identified in the phytochemical screening of the seed powder.
Discussions
The screening of aqueous and ethanolic extracts of Ricinodendron heudelotii seed for antifungal properties revealed antifungal activity against C albicans and no activity against A niger.SDA which was used as a positive control showed growth of the two organisms (table 3, figure 2). These results are similar to those obtained when similar work was done on the oil extract from the seeds [36] of the plant which has exhibited antifungal activity against C albicans.
The susceptibility test of the test organisms to the various extracts of Ricinodendron heudelotii seeds using agar dilution method showed that the aqueous extract does not inhibit the growth of the chosen isolates while total inhibition of the growth of C albicans was observed on the alcoholic extract and no inhibition of Aspergillus niger growth was observed.
The phytochemical screening revealed the presence of bioactive compounds which have been found to have antimicrobial activities in vitro. The results obtained showed the presence of bioactive compounds like resins and saponins while, alkaloids, glycosides, tannins and flavonoids were absent (table 4). However, it is known that the plant composition will depend on the geographical origin [37].
Resins were present in moderate amount. Similar work done on the stem and bark [10] have also indicated the presence of the compounds. Herbs that have resins have been reported for their antimicrobial activities and wound healing to animals and plants that secrete them. Saponins were also present in small amount. This is similar to work done on the stem bark [10] which has revealed presence of these chemicals. Studies have illustrated the beneficial effects on blood cholesterol levels, cancer, bone health and stimulation of the immune system [16].
Tannins were absent. This differs from works done on the bark [36] which has revealed presence of the chemical. Flavonoids, glycosides and alkaloids were absent in similar works done on the bark [36].
Conclusion
In deduction, plants are studied as potential disease controlling agents in humans as they are relatively safe, affordable and are easily accessible at a local level, such that they can offer an alternative treatment option to the conventional antibiotics. This study has allowed us to determine the antifungal activity of seed extracts of R heudelotii on C albicans and A niger. The results of these investigations show that the alcoholic extracts of ricinodendron heudelotii seeds have antifungal activity only against C albicans and the aqueous extract does not show any antifungal activity against the chosen isolates. It also revealed the presence of some phytochemical compounds (resins and saponins) with antimicrobial properties.
Recommendations
- To the ministry of public health: That more research should be done on medicinal plants in other to search new methods of treating common diseases among the community.
- To medical laboratory students: That somebody can carry out research on the antibacterial activities of the seeds.
- To the public: It is advised to confirm that the infection is actually C albicans before start taking the seeds.
- To researchers: Toxicity effects of the seeds should be studied.
Mcfarland standards
Original Mcfarland standards are made by mixing specified amounts of barium chloride and sulfuric acid together. Mixing the two compounds forms a barium sulfate precipitate, which causes turbidity in the solution. A 0.5 Mcfarland standard is prepared by mixing 0.05ml of 1.175% barium chloride dehydrate (BaCl2.2H2O), with 9.95 ml of 1% sulfuric acid (H2SO4).
There are Mcfarland standards prepared from suspensions of latex particles, which lengthens the shelf life and stability of the suspensions. The standard can be compaired visually to a suspension of bacteria in sterile saline or nutrient broth. If the bacterial suspension is too turbid, it can be diluted with more diluents. If the suspension is not turbit enough, more bacteria can be added.

