Anubha Bajaj*
Department of Histopathology, AB Diagnostics, New Delhi, India
*Corresponding author: Bajaj A, Department of Histopathology, AB Diagnostics, New Delhi, India
Citation: Bajaj A. (2022) Antediluvian and Remodeled-Early Gastric Carcinoma. Genesis J Surg Med. 1(2):1-5.
Received: December 8, 2022 | Published: December 29, 2022
Copyright© 2022 genesis pub by Bajaj A, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Abstract
Early gastric carcinoma (EGC) is denominated as a neoplasm confined to gastric mucosa or submucosa irrespective of regional lymph node metastasis. Early gastric carcinoma is associated with preceding amalgamation of precancerous lesions. Additionally, designated as mucosal or submucosal gastric carcinoma, early gastric carcinoma manifests tubular adenocarcinoma as a commonly discerned histologic subtype. Early gastric carcinoma can be appropriately discerned with endoscopy followed by confirmatory tissue sampling. Early gastric carcinoma exhibits excellent prognostic outcomes with 5-year proportionate survival at 90%. Regional lymph node metastasis is a critical prognostic factor contributing to disease associated survival. Appropriate distinction between well differentiated early gastric carcinoma and high-grade dysplasia or carcinoma and high-grade dysplasia or carcinoma in situ can be challenging.
Keywords
Gastric carcinoma; Cancer; Metastasis; Adenocarcinoma
Introduction
Early gastric carcinoma is preponderantly discerned within Asian population, possibly due to intensive screening. Average age of disease emergence is 62.2 years. A male predominance is observed with male to female proportion of 2.29:1 [1,2]. Early gastric carcinoma commonly occurs within diverse gastric regions as cardia, corpus or fundus, antrum, angularis or gastric pylorus.
Majority (90%) of instances of the multifactorial malignancy are sporadic. Familial or hereditary neoplasms are associated with various chromosomal anomalies as CDH1 genetic mutation discerned with hereditary diffuse gastric carcinoma or APC genetic mutation accompanying proximal polyposis [1,2]. Environmental factors such as smoking, elevated dietary intake of salt, consumption of preserved or smoked foods, meat and infection with Helicobacter pylori or Epstein Barr virus (EBV)may contribute to disease emergence [1,2]. Commencement of gastric epithelial injury due to environmental factors as Helicobacter pylori or Epstein Barr Virus (EBV) infection associated with chronic inflammation induces dysfunction of gastric stem cells within implicated zone with consequent dysregulated differentiation and emergence of gastric carcinoma [1,2]. Early gastric carcinoma is preceded by spectrum of precancerous lesions as atrophic or non-atrophic chronic gastritis inducing partial or complete intestinal metaplasia, low grade dysplasia, subsequent high grade dysplasia or carcinoma in situ and invasive gastric carcinoma. Over expression of PDL1 is observed within microsatellite instability (MSI) and Epstein-Barr virus (EBV) associated neoplasms [1,2]. Tumours depicting MSI appear prone to immune checkpoint blockade. Neoplasms associated with Epstein-Barr virus (EBV) may demonstrate Epstein-Barr encoding region (EBER) with in situ hybridization (ISH). Tumefaction with MSI can exemplify mismatch repair (MMR) genes upon cogent immunohistochemistry with protein analysis or microsatellite molecular evaluation [1,2]. Neoplasms with MSI characteristically demonstrate frequent hyper-methylation of MLH1 promotergene [1,2].
Early gastric carcinoma can commonly represent as an asymptomatic condition. Symptomatic instances exhibit nonspecific features as anaemia, epigastralgia or dyspeptic syndrome [1,2]. Upon endoscopy, early gastric carcinoma is classified as
1. type 0-I enunciates polypoid growth sub-classified into type 0-Ip demonstrating lesions with pedunculated growth type 0-Is exemplifying lesions with sessile growth.
2. type 0-II exhibiting non-polypoid lesions denominated as type 0-IIa depicting slightly elevated lesions type 0-IIb delineating flattened lesions type 0-IIc displaying slightly depressed lesions.
3. type 0-III demonstrating lesions with excavated growth.
4. Macroscopically, early gastric carcinoma is categorized into.
5. type 0-I comprised of protruding lesions.
6. type II composed of superficial neoplastic lesions further divided into.
7. type 0-IIa comprised of superficially elevated lesions type 0-IIb constituted of flattened lesions type 0-IIc enunciating superficially depressed lesions.
8. type 0-III exemplifying excavated lesions.
9. type IV comprised of infiltrating lesions associated with lateral tumour dissemination [1,2].
Histologically, early gastric carcinoma demonstrates significant architectural and cytological heterogeneity with concurrence of diverse histological patterns [1,2]. Contingent to predominant histological configuration, World Health Organization (WHO) 2010 defines distinctive histologic patterns of gastric carcinoma designated as tubular, papillary, mucinous and poorly cohesive along with signet ring cell carcinoma [1,2]. Histological assessment of early gastric carcinoma commonly depicts well differentiated adenocarcinoma with tubular or papillary architecture. Tubular adenocarcinoma is a frequently discerned histologic subtype of early gastric carcinoma. Grossly, tubular adenocarcinoma configures polypoid or fungating tumour mass [1,2]. Upon low power examination, glands may simulate alphabets W, H, Y or X. Neoplastic glands exhibit subtle architectural alterations.
Tumefaction demonstrates irregular, dilated, fused or branching tubules of variable magnitude. Intraluminal aggregates of mucus, nuclear or inflammatory debris are discerned [1,2]. Tumour cells depict enlarged, hyper chromatic, pleomorphic nuclei with prominent nucleoli, enhanced nuclei-cytoplasmic ratio and augmented mitotic activity with atypical mitotic configurations. Papillary adenocarcinoma incriminates elderly subjects, occurs within proximal gastric region and frequently depicts metastasis into hepatic parenchyma and regional lymph nodes [1,2]. Tumefaction demonstrates micro-papillary and tubular components. Characteristic epithelial projections with scaffolds of centric fibro-vascular core can be observed. Lesions akin to papillary adenocarcinoma depict finger-like projections confined to superimposed mucosal surface. Glandular articulations are layered with neoplastic columnar cells [1,2]. In contrast to tubular adenocarcinoma, aforesaid neoplasm enunciates extensive lymphatic and vascular invasion with regional lymph node metastasis and inferior prognostic outcomes.
Early gastric carcinoma demonstrating poorly cohesive neoplastic cells or occurring as signet ring cell carcinoma exemplify neoplastic cells configuring miniature aggregates or isolated cells with an absence of well-formed glandular articulations [1,2]. Characteristically, signet ring cells delineate a centric, optically clear, globoid accumulation or droplets of cytoplasmic mucin with an eccentric nucleus. Poorly cohesive early gastric carcinoma and signet ring cell carcinoma is comprised of an admixture of signet ring cells and non-signet ring cells [1,2]. Poorly cohesive, non-signet ring tumour cells morphologically simulate histiocytes, lymphocytes or plasma cells. Neoplastic cells configure irregular micro-trabeculae or lace-like, abortive glandular structures. Adjacent gastric wall appears macroscopically depressed or exhibits an ulcerated surface along with prominent desmoplastic reaction [1,2]. Antro-pyloric poorly cohesive and signet ring cell neoplasms with incrimination of serosa are associated with lymphatic and vascular invasion, regional lymph node metastasis and propensity to infiltrate duodenum through submucosa and subjacent serosa along with incrimination of subserosal and submucosal lymphatic spaces [1,2].
Immune reactivity to cytokeratin is beneficial in discerning morphologically occult signet ring cells invading the lamina propria. Neoplastic signet ring cells confined to gastric mucosa necessitate differentiation from benign pseudo-signet ring cells. Pseudo-signet ring cells may demonstrate cytological atypia or occasional mitoses [1,2]. However, pseudo-signet ring cells may not configure an invasive tumour pattern. Reticulin stain emphasises pseudo-signet ring cells confined to basement membrane along with an intact acinar architecture. Intra-mucosal signet ring cell carcinoma is associated with minimal possible regional lymph node metastasis [1,2]. Unlike carcinoma in situ, high grade dysplasia or intramucosal colonic carcinoma, early or intra-mucosal gastric carcinoma is associated with distant metastasis [1,2]. Generally, intra-mucosal gastric carcinoma enunciates invasion of singular neoplastic cells within lamina propria and prominent, fused neoplastic glands of variable dimension [1,2].
Mucinous adenocarcinoma exemplifies malignant epithelial cell layer typically intermingled with pools of extracellular mucin. Neoplastic component configures glandular architecture admixed with irregular cellular clusters. Occasional signet ring cells appear scattered within extracellular mucin pools [1,2]. Exceptional morphological subtypes of early gastric carcinoma occur as hepatoid adenocarcinoma, micro-papillary adenocarcinoma, gastric adenocarcinoma of fundic gland type, carcinoma with lymphoid stroma, adenosquamous carcinoma, squamous carcinoma, choriocarcinoma, parietal cell carcinoma, malignant rhabdoidtumor, mucoepidermoid carcinoma, Paneth cell carcinoma, undifferentiated carcinoma, mixed adeno-neuroendocrine carcinoma, endodermal sinus tumour, embryonal carcinoma, pure gastric yolk sac tumour oroncocytic adenocarcinoma [1,2].

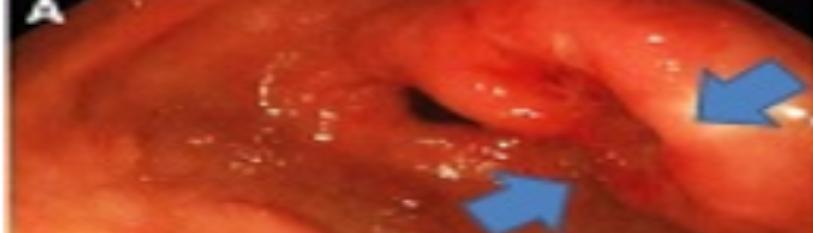
Figure 1: Early gastric carcinoma displaying mucosal erosions and ulceration along with configuration of elongated tubular and papillary mucosal excrescences layered with neoplastic epithelium with hyper-chromatic, pleomorphic nuclei [5].

Figure 2: Early gastric carcinoma depicting tubular and papillary configurations confined to mucosa layered with neoplastic cells with cellular and nuclear atypia, hyperchromatic and pleomorphic nuclei with prominent nucleoli [6].
TNM Classification of Gastric Carcinoma is Denominated as Primary Tumour
1. TX: Primary tumour cannot be assessed.
2. T0: No evidence of primary tumour.
3. Tis: Carcinoma in situ which expounds an intraepithelial tumour devoid of infiltration of lamina propria and exhibits high grade dysplasia.
4. T1: Tumour invades lamina propria, muscularis mucosae or submucosa and is categorized as
5. T1a: Tumour invades lamina propria or muscularis mucosae.
6. T1b: Tumour invades submucosa.
7. T2: Tumour invades muscularispropria.
8. T3: Tumour penetrates connective tissue subjacent to serosa with absence of metastasis into visceral peritoneum or adjacent viscera.
9. T4: Tumour invades serosa, visceral peritoneum or adjacent viscera and is categorized as.
10. T4a: Tumour invades serosa and visceral peritoneum.
11. T4b: Tumour invades adjacent anatomical structures or organs.
Regional Lymph Nodes
1. NX: Regional lymph nodes cannot be assessed.
2. N0: Regional lymph node metastasis absent.
3. N1: Metastasis within one or two regional lymph nodes.
4. N2: Metastasis within three to six regional lymph nodes.
5. N3: Metastasis within ≥7 regional lymph nodes which is categorised as.
6. N3a: Metastasis within 7 to 15 regional lymph nodes.
7. N3b: Metastasis within ≥ 16 regional lymph nodes
Regional lymph nodes are denominated by lymph nodes confined to greater curvature, lesser curvature or greater omentum, lesser omentum, right and left pericardial, cardio-oesophageal, supra-pyloric, gastroduodenal, infra-pyloric, gastroepiploic, left gastric artery, celiac artery, common hepatic artery, hepatoduodenal, portal or splenic artery and lymph nodes of splenic hilum.
Distant Metastasis
1. Oil M0: Distant metastasis absent.
2. M1: Distant metastasis present into distant organs as hepatic or pulmonary parenchyma, brain or parietal peritoneum [2,3].
Early gastric carcinoma is immune reactive to CK7, CK20, CDX2, MUC2, MUC5AC or CK5/6 [3,4]. Early gastric carcinoma is immune non reactive to oestrogen receptor (ER), progesterone receptor (PR), GCDFP-15, GATA3, E-cadherin or cytokeratin [3,4].
Early gastric carcinoma requires segregation from neoplasms such as metastatic lobular carcinoma breast, malignant melanoma or various epithelial alterations simulating signet ring cells or mesothelial cells, hyperplastic gastric polyp or gastric xanthom [3,4]. A Early gastric carcinoma can be appropriately discerned with endoscopy along with or devoid of dye spraying method [3,4]. Disease confirmation can be obtained with evaluation of surgical tissue samples [3,4]. Endoscopic ultrasound (EUS) is a pertinent technique for assessing depth of tumour invasion demonstrated by early gastric carcinoma.
Early gastric carcinoma is preferentially treated with gastrectomy along with lymphadenectomy. However, endoscopic resection is optimal in managing early gastric carcinomas depicting prognostic outcomes equivalent to surgical intervention [3,4]. An en bloc endoscopic resection is recommended as precise histological diagnosis is mandated for appropriate risk assessment of regional lymph node metastasis and possible localized tumour reoccurrence following ‘piecemeal resection’ [3,4].
Endoscopic resection may be singularly adopted in subjects with minimal possible regional lymph node metastasis and instances with ~tumour magnitude < 30 millimetres absence of lymphatic or vascular tumour invasion submucosal infiltration < 500 micrometres (μm) (sm1) absence of tumour within vertical resection margin [3,4]. Regional lymph node metastasis is a significant prognostic factor associated with progression of gastric carcinoma [3,4]. Specifically, 5 year survival associated with mucosal gastric carcinoma is 99.3% and submucosal neoplasm is 96.7% [3,4].
References
- Cho JH, Lee SH. (2022) Early gastric cancer presenting as a typical submucosal tumor cured by endoscopic submucosal dissection: A case report. World J Gastroenterol. 28(25):2994.
- Chiarello MM, Fico V, Pepe G, Tropeano G, Adams NJ, et al. (2022) Early gastric cancer: A challenge in Western countries. World J Gastroenterol. 28(7):693.
- Ferreira CN, Serrazina J, Marinho RT. (2022) Detection and Characterization of Early Gastric Cancer. Front Oncol. 12.
- Hashimoto R, Hamamoto H, Omori Y, Tanuma T. (2017) Early gastric cancer on submucosal heterotopic gastric glands. Gastrointest Endosc. 85(4):851-2.
- Image 1 Courtesy: Nature.com.
- Image 2 Courtesy: Pathology outlines.